As the ketogenic diet gains popularity, it’s important to have a balanced discussion regarding the merits of this diet and whom can benefit. Let me emphasize right out of the gate that ketogenic diets have significant therapeutic potential for some clinical pathologies. However, it is also a diet with inherent risk, as evidenced by the extensive list of adverse reactions occurring with high frequency reported in the scientific literature.
Table of Contents[Hide][Show]
For every anecdotal story of someone who has regained their health with a ketogenic diet, there’s a counterpoint story of someone who derailed their health with an identical diet. But, let’s not trade anecdotes. Instead, let’s review the huge collection of scientific papers—including results from clinical trials, case studies, and mechanistic studies—that reveal a dark side to the ketogenic diet. And that dark side is one that everyone needs to be aware of while they perform their own individual cost-benefit analysis to decide whether this dietary strategy is worth the risk for them.
A Brief History of Ketogenic Diets
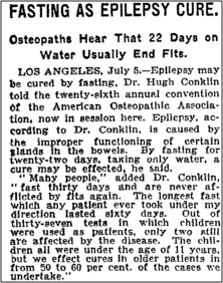
The origins of the ketogenic diet were the observations of Hippocrates in 500BC that fasting could reduce and even cure epileptic seizures (fasting is also a ketogenic state). However, it wasn’t until 1911 that the first scientific study on starvation as epilepsy treatment was published. Fasting has one very important barrier to rampant clinical use: it’s not sustainable. You can’t starve for the rest of your life and expect it to last very long.
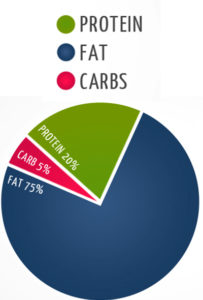
In 1921, Drs. Stanley Cobb and W.G. Lennox became the first to observe that control of seizures by fasting occurs via a change in body metabolism that can be induced either by absence of food or by a very low carbohydrate intake. And this observation led to the birth of a form of nutritional starvation, the ketogenic diet, a term coined by Dr. Russell Wilder who developed the diet as an alternative to fasting and demonstrated its comparable effectiveness in epilepsy. Dr. Wilder observed that the diet produced high levels of ketone bodies in the blood and hypothesized that a high fat, low carbohydrate diet would mimic the anticonvulsant effects of fasting because limited glucose supply would force fat to be metabolized into ketones, which could then be used as an alternative fuel by the brain (this hypothesis took decades to prove, and the more detailed mechanisms still remain to be elucidated). In 1925, Dr. Mynie Peterman, calculated the exact formula for nutritional ketosis, a daily diet comprised of: 10 – 15 grams of carbohydrate, 1 gram per kilogram of bodyweight of protein, and the rest fat (a macronutrient formula that is still within the narrow range used in ketogenic diet variations today).
Research into ketogenic diets went through many ups and downs over the decades that followed. Every time a new anticonvulsant drug for epilepsy was developed, interest in ketogenic diets waned under the presumption that it’s easier, and more tolerable, to take a pill every day compared to a severely restrictive diet that causes many intolerable side effects. However, interest was rekindled in the wake of professional and public recognition subsequent to: the popularity of the Atkins’ Diet Revolution; a Dateline report featuring a 2-year-old Charlie Abrahams whose intractable seizures were successfully stopped after he adopted a ketogenic diet; the creation of The Charlie Foundation which disseminates informational ketogenic diet videos to doctors and dietitians; and the movie Do No Harm. Since the late-1990s, research into ketogenic diets has expanded, including a series of long-term ketogenic diet studies for refractory epilepsy (epilepsy that does not respond to anticonvulsant drugs) and investigation of a variety of other applications.
Adverse Reactions to Ketogenic Diets
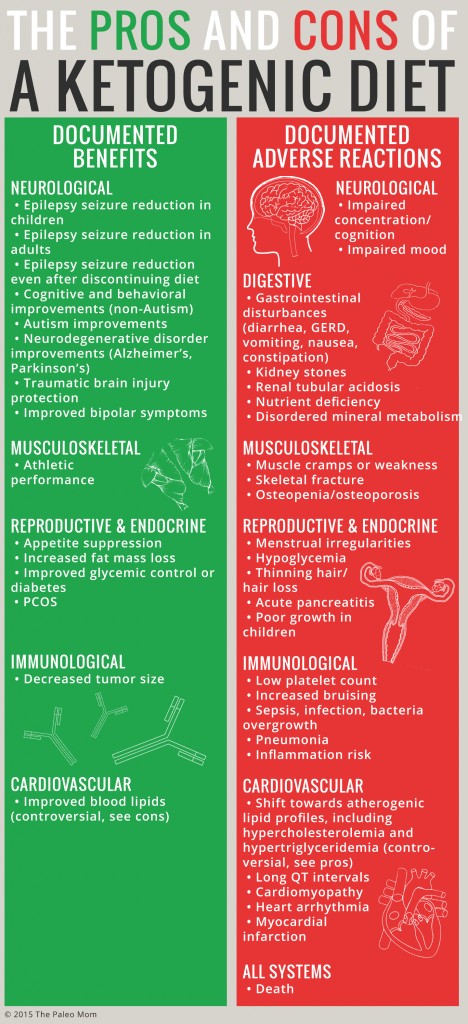
As the list of health conditions that may be at least partially alleviated by ketogenic diets increase (and which currently includes epilepsy, Alzheimer’s, Parkinson’s, autism, traumatic brain injury, bipolar disease, multiple sclerosis, PCOS, cancer, obesity, and diabetes), so too does a body of literature pointing to common side effects and potential adverse reactions.
An adverse reaction is an unwanted/unexpected and dangerous reaction to a therapeutic agent (as opposed to a side effect which is simply an unpleasant secondary effect). Adverse reactions to a ketogenic diet have been reported in the scientific literature. Yes, even including well-designed clinical trials performed and published very recently. In fact, the intolerability of side effects and adverse reactions is the primary reason for trial participants to drop out of ketogenic diet clinical trials (other reasons for trial drop-outs include ineffectiveness of the diet, and that the diet is too hard to follow).
Documented adverse reactions reported in long-term ketogenic studies (any study that allows for keto-adaptation, which takes up to a month) include:
- Gastrointestinal disturbances (diarrhea, vomiting, nausea, constipation, GER)
- Inflammation risk
- Sepsis, infection, bacteria overgrowth
- Pneumonia
- Acute pancreatitis
- Hypothyroidism
- Thinning hair/hair loss
- Menstrual irregularities and amenorrhea
- Hypoglycemia
- Increased cortisol
- Decreased testosterone
- Low platelet count
- Increased bruising
- Kidney stones
- Renal tubular acidosis
- Elevated liver enzymes
- Muscle cramps or weakness
- Impaired concentration/cognition
- Impaired mood
- Nutrient deficiency (most notably vitamin D, calcium, selenium, iron, potassium, phosphorous, vitamin C, magnesium and copper)
- Disordered mineral metabolism
- Poor growth in children
- Skeletal fracture
- Osteopenia/osteoporosis
- Shift towards atherogenic lipid profiles (including hypercholesterolemia and hypertriglyceridemia)
- Long QT intervals
- Cardiomyopathy
- Heart arrhythmia
- Myocardial infarction
- Death
Studies report adverse reaction frequency ranging from 10% to 100%, which may reflect variations in the rigor of reporting in addition to the actual incidence in different populations. The extensive list of documented adverse reactions indicate that the positive health effects of a ketogenic diet comes at a cost, one that may not be acceptable to many.
Nutrivore Weekly Serving Matrix
An easy-to-use and flexible weekly checklist
to help you maximize nutrient-density.
The Weekly Serving Matrix is very helpful! I’ve been eating along these lines but this really helps me know where to focus vs. which foods serve a more secondary role. It’s super helpful and has taken a lot of worry out of my meal planning. Thanks!
Jan
Mechanisms behind Adverse Reactions
The ketogenic diet is designed to mimic the biochemical changes that occur during starvation while meeting a nutritional threshold to avoid starving to death. So, it shouldn’t be a surprise that the above list of adverse reactions overlaps substantially with effects of starvation. Anorexia nervosa causes what is called starvation ketosis, the adverse effects of which include: abnormal blood counts, elevated liver enzymes, fatigue, dizziness or fainting, seizure, brittle nails, thinning hair, hair loss, amenorrhea, constipation, decreased appetite, dry skin, heart arrhythmia, osteoporosis, shift toward atherogenic lipid profiles, cold intolerance, hypercortisolemia, low blood pressure, dehydration, nutrient deficiency (including anemia, electrolyte imbalances, and deficiency diseases like scurvy), hypothyroidism, osteoporosis, bone fracture, kidney failure, edema, fatigue and lethargy, poor growth in children, and death. It’s a very similar list! So, which biological pathways are to blame for these adverse effects, whether due to true starvation or nutritional starvation?
It’s common for people following long-term ketogenic diets to fail oral glucose challenges, and animal studies of ketogenic diets provide an explanation for this: fatty-acid-induced insulin resistance. Proponents of ketogenic diets argue that it doesn’t matter if you’re insulin resistant if you don’t eat any carbs. But, this ignores the many, many physiologic roles of insulin that go beyond glucose homeostasis. In fact, the evidence is mounting that very low-carbohydrate diets, such as ketogenic diets, have negative effects on our health that are often analogous to insulin resistance, driven by the combination of fatty-acid-induced insulin resistance and insufficient insulin being secreted for normal signaling. See also The Case for More Carbs: Insulin’s Non-Metabolic Roles in the Human Body, 3 Ways to Regulate Insulin that Have Nothing to Do With Food and How Many Carbs Should We Eat?
Insulin activates an essential enzyme (type 2 deiodinase) which converts the thyroid prohormone T4 into the active hormone T3. In a recent study of pediatric epilepsy patients following a ketogenic diet for seizure control, participants had an overall decrease in free T3 and concurrent increase in TSH. A whopping 1 in 6 participants developed hypothyroidism requiring L-thyroxine medication within the first 6 months of the study! Of course, weight loss in general can reduce T3, however studies that have investigated the effect of weight loss via calorie restriction on thyroid function have not identified an increased risk of hypothyroidism (T3 and TSH remain within normal lab ranges).
Insulin increases the rate of transport of important amino acids into muscle tissue and increases muscle protein synthesis, while simultaneously reducing muscle protein breakdown. While all rapid weight loss diets result in loss of lean mass in addition to fat mass, when a hypocaloric ketogenic diet was pitted against a nonketogenic diet matched for calories and protein intake, there was greater protein sparing by the nonketogenic while total weight loss was equivalent between the two groups. The authors identify greater protein synthesis in the nonketogenic diet group as the mechanism for the protein sparing effect. Maintaining lean mass through weight loss is highly desirable and the body of evidence supports higher protein intake combined with physical activity is the best ways to achieve this goal.
Insulin regulates bone remodeling by driving osteoblast activity, the cell type responsible for bone mineralization. A recent study of epileptic children showed significant loss of bone mineral density (both whole body and lumbar spine) with two children sustaining fractures during the study period. These results confirmed an earlier study in which loss of bone mineral density was even higher, attributable to the fact that half of the children in that study started with lower-than-average bone mineral content. Only one study in adults has measured bone mineral density and no significant decline was observed over 5 years. However, effects of ketogenic diets on bone health are more readily observed in pediatric populations since children have higher rates of bone turnover and growth. See also The Paleo Diet for Skeletal Health
Insulin acts in the hippocampus to promote learning and memory, and to support synaptic plasticity. It also reduces microglia cell activity (resident immune cells in the brain). In a study of cognitive performance in young, healthy men following a short-term ketogenic diet, participants showed significantly reduced focused concentration, memory retrieval speed, and speed of visual information processing. In another study of overweight women following a long-term ketogenic diet, participants scored markedly lower on a neuropsychological test that requires higher order mental processing and flexibility (called the trail making task). Rodent studies have also shown detriment of ketogenic diets to cognition, impairing visual-spatial learning and memory.
Insulin regulates sex hormone levels by suppressing sex-hormone binding globulin and by enhancing estrogen and testosterone production. It also interacts with cortisol, growth hormone, glucagon, and hormone neurotransmitters like dopamine, serotonin and melatonin. In male athletes, some studies show reduced testosterone while others show increased; and in women with PCOS, ketogenic diets improved symptoms by decreasing testosterone. But, a significant percentage of young women following long-term ketogenic diets for epilepsy report amenorrhea (loss of menses). And, ketogenic diets suppress sex hormones in animal studies, including metabolites of progesterone and testosterone. Whether ketogenic diets are beneficial or harmful to hormone systems is likely very situation dependent. See also How Chronic Stress Leads to Hormone Imbalance
Insulin regulates the development and activity of many immune cells, generally improving the efficacy of the immune system while acting as an essential immune regulator. That’s why insulin resistance is associated with generalized inflammation; allergic, inflammatory, and autoimmune diseases; and reduced ability to fight off infection. One recent study showed an increase in C-reactive protein (a blood borne marker of inflammation) during weight loss with a low-carb, high-fat but not a high-carb diet. Another study in patients with rheumatoid arthritis showed no benefits to the cytokine IL-6 or T cell numbers and activation with a ketogenic diet. And, in an animal study of malignant glioma, a ketogenic diet increased the numbers and activation of effector cells (especially Th1) while decreasing the population of regulatory cells. Long-term studies of ketogenic diets in children and adolescents with epilepsy show a trend towards reduced white blood cells counts, while also reporting increased susceptibility to serious infection, affecting 10 to 15% of study participants. See also The Autoimmune Protocol
Scientists have discovered some other mechanisms explaining adverse reactions too. For example, ApoE4 carriers are genetically predisposed to higher cholesterol elevation during a high fat diet due to greater intestinal cholesterol resorption efficiency and more rapid hepatic clearance of dietary fat than non-ApoE4 carriers. About 25% of the population has at least one copy of ApoE4; this may explain the wide variation in changes to lipid profiles among ketogenic diet studies. (ApoE4 carriers are also at greater risk for heart disease, Alzheimer’s disease, and severe malaria. Interestingly, ApoE4 carriers experience no improvements in Alzeimer’s symptoms on a ketogenic diet.) Loss of bone mineral density in children following a ketogenic diet may also be attributed to acidosis. The inherent nutrient-deficiency of ketogenic diets explain many of the adverse effects; for example the cardiac complications can be caused by severe selenium deficiency. See also Genes to Know About: ApoE
Finally, substantial and problematic shifts in the gut microbiome are observed following a ketogenic diet, most likely attributable to severely inadequate fiber and starch intake and high saturated fat intake. In particular, ketogenic diets cause: a decrease in microbial diversity (diversity in the single most important aspect of a healthy gut microbiome); dramatically reduced (3- to 16-fold lower) levels of key probiotic bacteria (Bifidobacterium and Roseburia species, and Eubacterium rectale); and increased growth of Escherichia coli as well as Desulfovibrio species, a bacterial genus known to exacerbate the inflammatory condition of the gut mucosa. An unfavorable gut microbiome has been linked to conditions as wide-ranging as cancer, obesity and other metabolic problems, diabetes, heart disease, anxiety, depression, autism, autoimmunity, ulcers, IBD, liver disease, systemic infections, and more. See also How Ketogenic Diet Wreaks Havoc on Your Gut
Who Shouldn’t Do Keto:
Due to the capacity for ketogenic diets to greatly exacerbate certain conditions, it is not recommended for:
- Women considering getting pregnant (with the possible exception of those with PCOS): ketogenic diets can cause amenorrhea and loss of fertility
- People with hypothyroidism: ketogenic diets suppress thyroid function
- People with autoimmune disease (with the possible exception of multiple sclerosis and other autoimmune diseases affecting the central nervous system): the immune system changes induced by ketogenic diets are the opposite of what is desirable for autoimmune disease management
- Anyone with kidney stone risk factors: note that potassium citrate supplementation prevents a proportion of kidney stones due to ketogenic diet
- People without healthy livers: ketogenic diets put an additional strain on the liver and have been known to increase markers of liver damage
- People with one or two copies of the ApoE4 gene: you are much more likely to experience soaring triglycerides and LDL cholesterol with a ketogenic diet, and also less likely to benefit from it
- People with cardiovascular disease risk factors: If you do not have the ApoE4 gene, talk to your doctor about close monitoring of lipid profiles with a ketogenic diet because triglycerides and LDL cholesterol still can skyrocket
- People with osteopenia or osteoporosis: a ketogenic diet may increase loss of bone mineral density
- People with choline-deficiency: it increases risk for non-alcoholic fatty liver disease as a consequence of following a ketogenic diet
- People with selenium-deficiency: it increases risk for serious cardiac complications
- People with high cortisol, unmanaged stress, HPA-axis dysfunction: ketogenic diets increase cortisol secretion, which may worsen exacerbate stress-induced symptoms
- People with gut dysbiosis: ketogenic diets may exacerbate an already imbalanced gut microbiome
- People who can’t stick to it: because following a long-term ketogenic diet causes insulin resistance, one of the worst things is being “almost keto”.
And, for everyone opting to follow a ketogenic diet for therapeutic purposes, close medical supervision is strongly advised!
Deaths Caused by Ketogenic Diets
Five scientific papers have reported deaths connected to long-term ketogenic diets. Two of these papers are case studies, and the other three are papers derived from two separate clinical trials, all studies in epileptic children and adolescents. Some of the deaths can be attributed to extra complications from secondary conditions or accidents that befell the child during the course of the clinical trial; however, other deaths—from severe infection or heart disease—may be attributed directly to long-term ketosis.
Warning: the following summaries of deaths attributable to ketogenic diets may be upsetting to some readers.
Stewart WA, Gordon K, Camfield P. Acute pancreatitis causing death in a child on the ketogenic diet. J Child Neurol. 2001 Sep;16(9):682.
In this case report, a 9-year old girl following a ketogenic diet for Glut1 deficiency syndrome presented at the ER in a coma with “decreased respiratory effort and shock, requiring resuscitation.” She went into cardiac arrest and died. The postmortem examination revealed hemorrhagic pancreatitis, which the author attributes to hypertriglyceridemia from her ketogenic diet.
Kang HC, Chung DE, Kim DW, Kim HD. Early- and late-onset complications of the ketogenic diet for intractable epilepsy. Epilepsia. 2004 Sep;45(9):1116-23.
This study followed 129 pediatric and adolescent epilepsy patients following a ketogenic diet for an average of 6 years. Over the course of the study, 4 patients died, three of infection and one of cardiomyopathy. However, all four patients had additional health challenges. The patient who died of cardiomyopathy had pyruvate dehydrogenase deficiency (PDHD); the patient who died of lipoid pneumonia had a history of prenatal hypoxic brain insult; and, the two patients who died of sepsis had meconium aspiration syndrome and previous encephalitis.
Kang HC, Kim YJ, Kim DW, Kim HD. Efficacy and safety of the ketogenic diet for intractable childhood epilepsy: Korean multicentric experience. Epilepsia. 2005 Feb;46(2):272-9.
The dataset presented in this paper overlaps the above study, now including 199 children using ketogenic diets for epilepsy management for one year. An additional child died, this time for unknown reasons. The authors recommended an autopsy but the parents refused.
Bank IM, Shemie SD, Rosenblatt B, Bernard C, Mackie AS. Sudden cardiac death in association with the ketogenic diet. Pediatr Neurol. 2008 Dec;39(6):429-31. doi: 10.1016/j.pediatrneurol.2008.08.013.
This case study reports on two boys who died after following ketogenic diets for approximately 3 years. The first, an 11-year old, died of complications related to torsade de pointes (a dangerous tachyarrhythmia), with documented QT prolongation. He had normal QT intervals before starting the ketogenic diet and no congenital heart defect. His first-degree relatives were screened and had normal EKGs. The second, a 7-year old, also had a normal EKG before starting a ketogenic diet, but a follow-up revealed prolonged QT intervals and selenium deficiency. He was given an additional selenium supplement but died suddenly at home one month later.
Suo C, Liao J, Lu X, Fang K, Hu Y, Chen L, Cao D, Huang T, Li B, Li C. Efficacy and safety of the ketogenic diet in Chinese children. Seizure. 2013 Apr;22(3):174-8
This study followed 317 children on a ketogenic diet for epilepsy for a year. Over the course of the study, 10 children died, although 3 of those withdrew from the study prior to their deaths and 1 died after falling from height. Of the remaining children, 2 died of status epilepticus, 2 died of pneumonia, and the cause of death was undisclosed for the last 2 cases.
Are these deaths relevant to the average person looking to improve their health with a ketogenic diet approach? Because the overwhelming majority of the long-term ketogenic diet studies have thus far been performed in the context of epilepsy, this is the field of research that has most thoroughly reported adverse reactions. Certainly, these kids belong to a more sensitive population, but that doesn’t mean that these reports don’t provide extremely useful information, or a warning worth heeding. After all, it’s not typically the robust healthy person who experiments with ketogenic diets to improve their health—and while death is probably very unlikely, the potential harm to other body systems (cardiovascular, immune, endocrine, digestive) remains. Close medical supervision while following a ketogenic diet is clearly indicated.
Select Scientific Studies Reporting Adverse Reactions
The high frequency of adverse reactions are often glossed over in media reports and website articles, which focus on efficacy of the ketogenic diet (which this article does not dispute). To give you a sense of the frequency and severity of adverse reactions to long-term ketogenic diets, along with population relevance, the following is a sampling of clinical trials reporting adverse effects. Note, the rigor of adverse event reporting varies dramatically from study to study.
Sirven, et al., 1999: “The ketogenic diet for intractable epilepsy in adults: preliminary results”
Duration: 8+ months
Population: 11 adults (9 women, 2 men) ages 19 – 45 years, treated with ketogenic diet of 4:1 fat/non-fat ratio; no medication changes while on diet
Adverse Effects:
- 100% of participants had gastrointestinal complaints such as constipation and bloating
- 100% of female patients had menstrual irregularities (missed or irregular cycles)
- 73% reported hunger
- 18% had impaired concentration
- Unfavorable blood lipid effects: triglycerides and LDL cholesterol both increased in nearly all patients. Cholesterol/HDL ratio rose from 3.8 at baseline to 5.02 (more atherogenic).
- One patient whose seizures responded poorly ended diet after 5 months, and another 5 months after termination, had a myocardial infarction (cholesterol/HDL ratio had risen in this patient).
Coppola, et al., 2002: “The ketogenic diet in children, adolescents and young adults with refractory epilepsy: an Italian multicentric experience”
Duration: 1 – 18 months
Population: 56 young patients, ages 1 – 23, with drug resistant epilepsy; placed on 4:1 ketogenic diet with calories adjusted to avoid weight loss or gain
Adverse Effects: Adverse reactions in 57% of patients
- 18% had severe constipation
- 16% had irritability
- 16% had drowsiness
- 14% patients had hyperlipidemia
- 11% had episodes of vomiting
- 7% had hypoglycemia episodes
- 6% had recurrent abdominal pain
- 5% had acute episodic diarrhea
- 4% had oral candidosis
- 4% had hyporexia
- 2% had severe acidosis and ketonemia
Kwiterovich, et al., 2003: “Effect of a High-Fat Ketogenic Diet on Plasma Levels of Lipids, Lipoproteins, and Apolipoproteins in Children”
Duration: 6 months, prospective cohort study
Population: 141 children and young adults, aged 4 months – 20 years, placed on ketogenic diet for seizure control
Adverse Effects:
- On average total cholesterol, LDL, VLDL, non-HDL, triglycerides and total apoB all significantly increased. Average HDL cholesterol decreased significantly.
- After embarking on diet, only 1 in 6 had cholesterol or triglyceride levels in the acceptable range for their age group
Mady, et al., 2003: “The Ketogenic Diet: Adolescents Can Do It, Too”
Duration: 6 months; average diet duration 1.2 years
Population: 45 epileptics, aged 12 – 19 years
Adverse Effects:
- 45% of female participants reported menstrual problems
- 67% experienced amenorrhea
- 33% experienced delayed puberty
- 11% experienced menorrhagia after diet discontinuation
- 89% had menses return to normal after diet discontinuation
- 4% had increased bruising or bleeding
- 4% had reported thinning hair and/or hair loss
Yancy, et al., 2004: “A Low-Carbohydrate, Ketogenic Diet versus a Low-Fat Diet To Treat Obesity and Hyperlipidemia: A Randomized, Controlled Trial”
Duration: 24 weeks
Population: 120 overweight, hyperlipidemic adults placed on either ketogenic or low-fat, low-cholesterol diet
Adverse Effects:
- 2 people dropped out due to elevated LDL-C (rise of 184 to 283mg/dL at 3 months, and rise of 182 to 219 mg/dL at 1 month)
- 30% had elevated LDL-C of at least 10%
- 68% had constipation
- 60% experienced headaches
- 38% experienced halitosis
- 35% had muscle cramps
- 23% had diarrhea
- 25% complained of general weakness
Kang, et al., 2004: “Early- and late-onset complications of the ketogenic diet for intractable epilepsy”
Duration: At least 1 year; 6 year average
Population: 129 patients at epilepsy center (infants, children, and adolescents); ketogenic diet supplemented with multivitamins, calcium (30 mg per kg of body weight daily), vitamin D (40 IU per kg of body weight daily), L-Carnitine (66 mg per kg of body weight daily)
Long-Term Adverse Effects (beyond 4 weeks): 17% of patients stopped diet due to serious complications
- 28% had late-onset gastrointestinal discomfort
- 20% had hypertriglyceridemia
- 19% hypercholesterolemia
- 15% had osteopenia
- 11% had hypomagnesemia
- 8% had hyperuricemia
- 5% had hepatitis
- 5% had lipoid aspiration pneumonia
- 4% had hypoproteinemia
- 3% had renal stones
- Other adverse events reported included secondary hypocarnitinemia, iron-deficiency anemia, low HDL concentrations, symptomatic hypoglycemia, and cardiomyopathy
- 1 patient discontinued diet because of persistent and uncontrollable hypertriglyceridemia of over 1,000 mg/dL; 12 patients persisted despite triglycerides over 500 mg/dL
- 4 patients died
Johnston, et al., 2006: “Ketogenic low-carbohydrate diets have no metabolic advantage over nonketogenic low-carbohydrate diets”
Duration: 6 weeks
Population: 20 adults assigned to either ketogenic (5% carbohydrate) or nonketogenic diet; 24-hour intakes strictly controlled
Adverse Effects:
- Inflammatory risk (ratio of arachidonic to eicosapentaenoic acid in plasma phospholipids) more adversely affected by ketogenic diet than by nonketogenic diet
- HDL cholesterol fell average of 9%
- “Perceptions of vigor” lower on ketogenic diet
- One participant on ketogenic diet developed heart arrhythmias on first week of diet and dropped out
- 9% of the variation in LDL cholesterol was directly related to blood ketone concentrations, and several participants following the ketogenic diet had marked increases in LDL cholesterol
Sampath, et al., 2007: “Kidney stones and the ketogenic diet: Risk factors and prevention”
Duration: Retrospective cohort study of all children in clinic from 2000 through 2005; several years+ for each child
Population: 197 children
Adverse Effects:
- 7% developed kidney stones after a median of 7 months
- Potassium citrate therapy was preventative: 3.2% of children taking it had kidney stones versus 10% of those who did not
- Overall: Kidney stone prevalence ranges from 3 to 10% of ketogenic dieters, versus 1 in several thousand in general population
Mosek, et al., 2009: “Ketogenic diet treatment in adults with refractory epilepsy: A prospective pilot study”
Duration: 1 – 12 weeks follow-up; only two patients completed all 12 weeks
Population: 9 adult patients enrolled (ages 18 – 45) placed on 90% fat ketogenic diet
Adverse Effects:
- 78% dropped out due to feelings of hunger, lack of efficacy, or diarrhea
- Blood lipid changes:
- 26% rise in total cholesterol after 4 – 7 weeks and 33% rise after 11 – 12 weeks (three people)
- LDL rose by 32% and 54% in those same time frames, with no HDL change
- One patient’s triglycerides rose to 834 mg/dL and he subsequently terminated the diet
Goday A, et al., 2016. “Short-term safety, tolerability and efficacy of a very low-calorie-ketogenic diet interventional weight loss program versus hypocaloric diet in patients with type 2 diabetes mellitus.”
Duration: 4 months
Population: 45 adults assigned to low-calorie ketogenic diet (c.f. 44 participants assigned to standard low calorie diet)
Adverse Effects:
- 18% had constipation
- 13% had orthostatic hypotension
- 7% had nausea
- 4% had headaches
- 4% had hair loss
- Other reported reactions were asthenia, vomiting, myalgia and edema
Lin A, et al., 2017: “Complications During Ketogenic Diet Initiation: Prevalence, Treatment, and Influence on Seizure Outcomes.”
Duration: 3 months
Population: 158 children, mean age 4.6 years
Adverse Effects:
- 80% reported adverse effects of any kind
- 42% reported gastrointestinal upset
- 28% experienced hypoglycemia to levels <40 mg/dL
- 16% had documented repeated hypoglycemia to <40 mg/dL
- 10% had hypoglycemia to <30 mg/dL
- Other reported reactions included constipation, acidosis, sinusitis, fussiness or mood change, and over-ketosis
Duration: 6 weeks
Population: 42 healthy adults
Adverse Effects:
- Total cholesterol increased by 4.7%
- LDL cholesterol increased by 10.7%
- Two participants were unable to continue the diet due to persistent side effects (gastrointestinal complaints and headache)
Di Lorenzo C, et al., 2018: “Efficacy of Modified Atkins Ketogenic Diet in Chronic Cluster Headache: An Open-Label, Single-Arm, Clinical Trial.”
Duration: 12 weeks
Population: 18 drug-resistant chronic cluster headache patients, age 25 to 55
Adverse Effects:
- 6% reported hair loss
- 6% reported abdominal bloating
Baby N, et al., 2018: “A pragmatic study on efficacy, tolerability and long term acceptance of ketogenic diet therapy in 74 South Indian children with pharmacoresistant epilepsy.”
Duration: Median of 10.43 months
Population: 74 children with epilepsy, aged 0-18 years
Adverse Effects:
- 5% had to discontinue the diet due to major adverse events
- 3% had severe diarrhea
- 3% had lipoid pneumonia, resulting in prolonged ICU care and discontinuation of the diet
- 4% had hypoglycemia
- Other reported adverse reactions included persistent hyperammonemia, diarrhea and vomiting, renal calculi, constipation, and aspiration pneumonia
No, the Inuit Are Not Ketogenic Hunter-Gatherers
 This seems like a good opportunity to clear up a common myth about the Inuit: no, they are no hunter-gatherer cultures that live in sustained ketosis due to their very low carbohydrate intake, and no, they do not provide evidence that long-term ketosis is safe for humans.
This seems like a good opportunity to clear up a common myth about the Inuit: no, they are no hunter-gatherer cultures that live in sustained ketosis due to their very low carbohydrate intake, and no, they do not provide evidence that long-term ketosis is safe for humans.
The Inuit have a gene mutation that prevents them from entering ketosis (instead, they’re able to burn long-chain fatty acids for energy, which may help increase their body temperature in their notoriously cold climate). The mutation occurs on the CPT1A gene (it’s the P479L variant), which encodes a major rate-limiting enzyme for long-chain fatty acid oxidation. Interestingly, this mutation evolved in multiple unrelated populations, the common denominator being cold climate and low access to carbohydrate, implying that it is advantageous to survival. The vast majority of the Inuit population carry the CPT1A, P479L variant: approximately 70% of Inuit are homozygous for the gene variant, 24% are heterozygous, and only about 6% do not carry the gene.
The CPT1A, P479L variant makes it so that any situation that would require relying on ketones (such as fasting) becomes virtually lethal to the Inuit. Because the mutation is linked to failure to generate ketones in infancy, it may be responsible for the statistically higher rate of infant mortality in Inuit population. It also solves the previous mysteries as to why no elevated ketone levels have been found among the Inuit, and why they have normal glucose tolerance on their traditional diet (something not seen during ketosis, due to the physiological insulin resistance that occurs).
CPT1a gene
What’s more, even if the Inuit didn’t possess their unique gene mutation, their diet still wouldn’t be ketogenic due to its super high protein content (averaging 280 grams per day), since protein can be converted to glucose through the process of gluconeogenesis (and thus curtail ketosis) and due to some unique sources of dietary carbohydrates (up to 20% of total calories) like the glycogen in whale blubber. In reality, we have no evidence of any hunter-gatherer society surviving on a ketogenic diet—even among the handful living in environments where it would theoretically be possible!
The Inuit (on their traditional diet) definitely had a huge intake of high-fat animal foods, but they also went to great lengths to gather amazingly nutrient-dense plants whenever they were available (as well as preserve them for year-round use). Those included kelp, algae, and other seaweeds (which are super high in chlorophyll), plankton from whales’ stomachs, fireweed, sorrel grass, flower blossoms preserved in seal oil, mosses, a variety of berries, ground nuts, lichens, willow leaves, sourdock, scurvygrass, roseroot, numerous other weeds and greens, tubers, starchy corms (which the Inuit collected from caches made by tundra mice and voles), and the partially digested stomach contents of caribou and other land animals (considered a delicacy, and usually consisting of a variety of lichens, blueberry leaves and shoots, horsetails, grasses, birch, willow, and other plants the animals had grazed on).
That said, while there aren’t any true ketogenic hunter-gatherer societies, there certainly are plenty of hunter-gatherers who consume fewer carbohydrates (and more fat) than recommended by the USDA and other official health organizations—and who remain enviably free from obesity and chronic disease. The Nukak, Onge, Anbarra, Ache, Hiwi, Arnhem, and !Kung tribes are all examples of such hunter-gatherer groups thriving on a higher fat intake without suffering our modern plague of diseases.
Although long-term ketogenic diets don’t have any historical precedent (at least among entire populations), there are certainly examples of a higher fat intake (compared to what’s considered normal in industrialized nations) sustaining indigenous groups and protecting against disease. In other words, consuming a higher-fat diet of high quality, nutrient-dense foods can definitely be compatible with human health!
Cost Benefit Analysis
When you read reports expounding on the benefits of a ketogenic diet, purporting that there is no risk involved or at least no risk for most of us, the origin of this dogma is either a selective reading of the science or a bias-motivated dismissal of any scientific studies to the contrary of this narrative. For example, you may see adverse reactions described as “minor” and “transient” or attributable in some way to “people doing keto wrong”, statements that are not actually substantiated by the scientific literature (although, those might be fair appraisals of side effects reported in short-term studies).
The ketogenic diet is different from other diets because it’s designed to emulate starvation, taking advantage of the biochemical benefits of starvation for certain body systems (mainly neurological, but this is also why weight loss is a common result of a ketogenic diet) while tolerating the detriments to other body systems (such as endocrine and immune systems). In this regard, the ketogenic diet more closely resembles a drug than a diet (in fact, many of the same pathways activated by a ketogenic diet are manipulated by anticonvulsant drugs), which should not be a deterrent in itself, but rather a caution to read the fine print and talk to your doctor before embarking on this course—especially if going “off-label” and using a ketogenic diet for purposes that have not yet been well studied.
Certainly some of the adverse reactions can be prevented with careful choice of foods and/or targeted supplementation. It’s also important to emphasize that the documented adverse events point to a long list of tests that should be performed regularly by a supervising health professional to monitor for any potential detriment in the event that there are compelling reasons to undertake a ketogenic diet.
It is important to be fully aware of the potential for adverse reactions when weighing a ketogenic diet versus other diets or therapeutic options. See also Modifying Paleo to Meet Your Goals
Citations
Abdulla H, Smith K, Atherton PJ, Idris I. Role of insulin in the regulation of human skeletal muscle protein synthesis and breakdown: a systematic review and meta-analysis. Diabetologia. 2016 Jan;59(1):44-55. doi: 10.1007/s00125-015-3751-0. Epub 2015 Sep 24.
Ahn WK, Park S, Kim HD. Protein-Losing Enteropathy as a Complication of the Ketogenic Diet. Yonsei Med J. 2017 Jul;58(4):891-893. doi: 10.3349/ymj.2017.58.4.891.
Allan, S. (1933). The Ketogenic Diet in Epilepsy. Journal of Mental Science, 79(327), 677-687. doi:10.1192/bjp.79.327.677
Arneth BM. Activation of CD4 and CD8 T cell receptors and regulatory T cells in response to human proteins. PeerJ. 2018 Mar 9;6:e4462. doi: 10.7717/peerj.4462. eCollection 2018.
Arslan N, Guzel O, Kose E, Yılmaz U, Kuyum P, Aksoy B, Çalık T. Is ketogenic diet treatment hepatotoxic for children with intractable epilepsy? Seizure. 2016 Dec;43:32-38. doi: 10.1016/j.seizure.2016.10.024. Epub 2016 Nov 13.
Arslan N, Kose E, Guzel O. The Effect of Ketogenic Diet on Serum Selenium Levels in Patients with Intractable Epilepsy. Biol Trace Elem Res. 2017 Jul;178(1):1-6. doi: 10.1007/s12011-016-0897-7. Epub 2016 Nov 21.
Azevedo de Lima P, Baldini Prudêncio M, Murakami DK, Pereira de Brito Sampaio L, Figueiredo Neto AM, Teixeira Damasceno NR. Effect of classic ketogenic diet treatment on lipoprotein subfractions in children and adolescents with refractory epilepsy. Nutrition. 2017 Jan;33:271-277. doi: 10.1016/j.nut.2016.06.016. Epub 2016 Jul 26.
Baby N, Vinayan KP, Pavithran N, Grace Roy A. A pragmatic study on efficacy, tolerability and long term acceptance of ketogenic diet therapy in 74 South Indian children with pharmacoresistant epilepsy. Seizure. 2018 May;58:41-46. doi: 10.1016/j.seizure.2018.03.020. Epub 2018 Mar 21.
Ballaban-Gil K, Callahan C, O’Dell C, Pappo M, Moshé S, Shinnar S. Complications of the ketogenic diet. Epilepsia. 1998 Jul;39(7):744-8.
Bank IM, Shemie SD, Rosenblatt B, Bernard C, Mackie AS. Sudden cardiac death in association with the ketogenic diet. Pediatr Neurol. 2008 Dec;39(6):429-31. doi: 10.1016/j.pediatrneurol.2008.08.013.
Bergeron N, Havel RJ. Prolonged postprandial responses of lipids and apolipoproteins in triglyceride-rich lipoproteins of individuals expressing an apolipoprotein epsilon 4 allele. J Clin Invest. 1996 Jan 1;97(1):65-72.
Bergqvist AG, Chee CM, Lutchka L, Rychik J, Stallings VA. Selenium deficiency associated with cardiomyopathy: a complication of the ketogenic diet. Epilepsia. 2003 Apr;44(4):618-20.
Bergqvist AG, Schall JI, Stallings VA, Zemel BS. Progressive bone mineral content loss in children with intractable epilepsy treated with the ketogenic diet. Am J Clin Nutr. 2008 Dec;88(6):1678-84. doi: 10.3945/ajcn.2008.26099.
Berry-Kravis E, Booth G, Taylor A, Valentino LA. Bruising and the ketogenic diet: evidence for diet-induced changes in platelet function. Ann Neurol. 2001 Jan;49(1):98-103.
Bertoli, S., Trentani, C., Ferraris, C., De Giorgis, V., Veggiotti, P., Tagliabue, A. Long-term effects of a ketogenic diet on body composition and bone mineralization in GLUT-1 deficiency syndrome: a case series. Nutrition. 2014 Jun;30(6):726-8. doi: 10.1016/j.nut.2014.01.005. Epub 2014 Jan 29.
Best TH, Franz DN, Gilbert DL, Nelson DP, Epstein MR. Cardiac complications in pediatric patients on the ketogenic diet. Neurology. 2000 Jun 27;54(12):2328-30.
Brinkworth GD, Buckley JD, Noakes M, Clifton PM, Wilson CJ. Long-term effects of a very low-carbohydrate diet and a low-fat diet on mood and cognitive function. Arch Intern Med. 2009 Nov 9;169(20):1873-80. doi: 10.1001/archinternmed.2009.329.
Chang CK, Borer K, Lin PJ. Low-Carbohydrate-High-Fat Diet: Can it Help Exercise Performance? J Hum Kinet. 2017 Mar 12;56:81-92. doi: 10.1515/hukin-2017-0025. eCollection 2017 Feb.
Clemente, FJ et al. “A Selective Sweep on a Deleterious Mutation in CPT1A in Arctic Populations” Am J Hum Genet. 2014 Nov 6;95(5):584-589. doi: 10.1016/j.ajhg.2014.09.016. Epub 2014 Oct 23.
Coppola G, Natale F, Torino A, Capasso R, D’Aniello A, Pironti E, Santoro E, Calabrò R, Verrotti A. The impact of the ketogenic diet on arterial morphology and endothelial function in children and young adults with epilepsy: a case-control study. Seizure. 2014 Apr;23(4):260-5. doi: 10.1016/j.seizure.2013.12.002. Epub 2013 Dec 12.
Coppola G, Veggiotti P, Cusmai R, Bertoli S, Cardinali S, Dionisi-Vici C, Elia M, Lispi ML, Sarnelli C, Tagliabue A, Toraldo C, Pascotto A. The ketogenic diet in children, adolescents and young adults with refractory epilepsy: an Italian multicentric experience. Epilepsy Res. 2002 Feb;48(3):221-7.
Corcoran AC and Rabinowitch IM. “A Study of the Blood Lipoids and Blood Protein in Canadian Eastern Arctic Eskimos” Biochem J. 1937 Mar; 31(3): 343–348.
Costa Rosa LF, Safi DA, Cury Y, Curi R. The effect of insulin on macrophage metabolism and function. Cell Biochem Funct. 1996 Mar;14(1):33-42.
Daka B, Rosen T, Jansson PA, Råstam L, Larsson CA, Lindblad U. Inverse association between serum insulin and sex hormone-binding globulin in a population survey in Sweden. Endocr Connect. 2012 Nov 19;2(1):18-22. doi: 10.1530/EC-12-0057. Print 2013 Mar 1.
David LA, Maurice CF, Carmody RN, Gootenberg DB, Button JE, Wolfe BE, Ling AV, Devlin AS, Varma Y, Fischbach MA, Biddinger SB, Dutton RJ, Turnbaugh PJ. Diet rapidly and reproducibly alters the human gut microbiome. Nature. 2014 Jan 23;505(7484):559-63. doi: 10.1038/nature12820. Epub 2013 Dec 11.
Di Lorenzo C, Coppola G, Di Lenola D, Evangelista M, Sirianni G, Rossi P, Di Lorenzo G, Serrao M, Pierelli F. Efficacy of Modified Atkins Ketogenic Diet in Chronic Cluster Headache: An Open-Label, Single-Arm, Clinical Trial. Front Neurol. 2018 Feb 12;9:64. doi: 10.3389/fneur.2018.00064. eCollection 2018.
Doksöz Ö, Çeleğen K, Güzel O, Yılmaz Ü, Uysal U, İşgüder R, Çeleğen M, Meşe T. The Short-Term Effects of Ketogenic Diet on Cardiac Ventricular Functions in Epileptic Children. Pediatr Neurol. 2015 Sep;53(3):233-237.e1. doi: 10.1016/j.pediatrneurol.2015.06.009. Epub 2015 Jun 18.
Elia M, Klepper J, Leiendecker B, Hartmann H. Ketogenic Diets in the Treatment of Epilepsy. Curr Pharm Des. 2017;23(37):5691-5701. doi: 10.2174/1381612823666170809101517.
Ellis RW. Some Effects of a Ketogenic Diet. Arch Dis Child. 1931 Oct;6(35):285-92.
Fischer HJ, Sie C, Schumann E, Witte AK, Dressel R, van den Brandt J, Reichardt HM. The Insulin Receptor Plays a Critical Role in T Cell Function and Adaptive Immunity. J Immunol. 2017 Mar 1;198(5):1910-1920. doi: 10.4049/jimmunol.1601011. Epub 2017 Jan 23.
Fraser DA, Thoen J, Bondhus S, Haugen M, Reseland JE, Djøseland O, Førre O, Kjeldsen-Kragh J. Reduction in serum leptin and IGF-1 but preserved T-lymphocyte numbers and activation after a ketogenic diet in rheumatoid arthritis patients. Clin Exp Rheumatol. 2000 Mar-Apr;18(2):209-14.
Fraser DA, Thoen J, Djøseland O, Førre O, Kjeldsen-Kragh J. Serum levels of interleukin-6 and dehydroepiandrosterone sulphate in response to either fasting or a ketogenic diet in rheumatoid arthritis patients. Clin Exp Rheumatol. 2000 May-Jun;18(3):357-62.
Fulzele K, Clemens TL. Novel functions for insulin in bone. Bone. 2012 Feb;50(2):452-6. doi: 10.1016/j.bone.2011.06.018. Epub 2011 Jun 24.
Garbow JR, Doherty JM, Schugar RC, Travers S, Weber ML, Wentz AE, Ezenwajiaku N, Cotter DG, Brunt EM, Crawford PA. Hepatic steatosis, inflammation, and ER stress in mice maintained long term on a very low-carbohydrate ketogenic diet. Am J Physiol Gastrointest Liver Physiol. 2011 Jun;300(6):G956-67. doi: 10.1152/ajpgi.00539.2010. Epub 2011 Mar 31.
Goday A, Bellido D, Sajoux I, Crujeiras AB, Burguera B, García-Luna PP, Oleaga A, Moreno B, Casanueva FF. Short-term safety, tolerability and efficacy of a very low-calorie-ketogenic diet interventional weight loss program versus hypocaloric diet in patients with type 2 diabetes mellitus. Nutr Diabetes. 2016 Sep 19;6(9):e230. doi: 10.1038/nutd.2016.36.
Greenberg, CR et al., “The paradox of the carnitine palmitoyltransferase type Ia P479L variant in Canadian Aboriginal populations” Mol Genet Metab. 2009 Apr;96(4):201-7. doi: 10.1016/j.ymgme.2008.12.018. Epub 2009 Feb 13.
Groesbeck DK, Bluml RM, Kossoff EH. Long-term use of the ketogenic diet in the treatment of epilepsy. Dev Med Child Neurol. 2006 Dec;48(12):978-81.
Hahn TJ, Halstead LR, DeVivo DC. Disordered mineral metabolism produced by ketogenic diet therapy. Calcif Tissue Int. 1979 Aug 24;28(1):17-22.
Hall KD, Chen KY, Guo J, Lam YY, Leibel RL, Mayer LE, Reitman ML, Rosenbaum M, Smith SR, Walsh BT, Ravussin E. Energy expenditure and body composition changes after an isocaloric ketogenic diet in overweight and obese men. Am J Clin Nutr. 2016 Aug;104(2):324-33. doi: 10.3945/ajcn.116.133561. Epub 2016 Jul 6.
Hall KD, Chung ST. Low-carbohydrate diets for the treatment of obesity and type 2 diabetes. Curr Opin Clin Nutr Metab Care. 2018 Apr 18. doi: 10.1097/MCO.0000000000000470.
Hallböök T, Ji S, Maudsley S, Martin B. The effects of the ketogenic diet on behavior and cognition. Epilepsy Res. 2012 Jul;100(3):304-9. doi: 10.1016/j.eplepsyres.2011.04.017. Epub 2011 Aug 27.
Hawkes CP, Levine MA. Ketotic hypercalcemia: a case series and description of a novel entity. J Clin Endocrinol Metab. 2014 May;99(5):1531-6. doi: 10.1210/jc.2013-4275. Epub 2014 Feb 25.
Heinbecker, P. “Studies on the Metabolism of Eskimos” The Journal of Biological Chemistry 1928 Dec;80, 461-475.
Henderson ST, Vogel JL, Barr LJ, Garvin F, Jones JJ, Costantini LC. Study of the ketogenic agent AC-1202 in mild to moderate Alzheimer’s disease: a randomized, double-blind, placebo-controlled, multicenter trial. Nutr Metab (Lond). 2009 Aug 10;6:31. doi: 10.1186/1743-7075-6-31.
Ho, K-J et al. “Alaskan Arctic Eskimo: Responses to a Customary High Fat Diet” Am J Clin Nutr. 1972 Aug;25(8):737-45.
Holloway CJ, Cochlin LE, Emmanuel Y, Murray A, Codreanu I, Edwards LM, Szmigielski C, Tyler DJ, Knight NS, Saxby BK, Lambert B, Thompson C, Neubauer S, Clarke K. A high-fat diet impairs cardiac high-energy phosphate metabolism and cognitive function in healthy human subjects. Am J Clin Nutr. 2011 Apr;93(4):748-55. doi: 10.3945/ajcn.110.002758. Epub 2011 Jan 26.
Hoyt CS, Billson FA. Optic neuropathy in ketogenic diet. Br J Ophthalmol. 1979 Mar;63(3):191-4.
Husain Z, Seth P, Sukhatme VP. Tumor-derived lactate and myeloid-derived suppressor cells: Linking metabolism to cancer immunology. Oncoimmunology. 2013 Nov 1;2(11):e26383. Epub 2013 Oct 9.
Ismayilova N, Leung MA, Kumar R, Smith M, Williams RE. Ketogenic diet therapy in infants less than two years of age for medically refractory epilepsy. Seizure. 2018 Apr;57:5-7. doi: 10.1016/j.seizure.2018.02.014. Epub 2018 Mar 2.
Jáuregui-Garrido B, Bolaños-Ríos P, Santiago-Fernández MJ, Jaúregui-Lobera I. Lipid profile and cardiovascular risk in anorexia nervosa; the effect of nutritional treatment. Nutr Hosp. 2012 May-Jun;27(3):908-13. doi: 10.3305/nh.2012.27.3.5752.
Jin HY. Prevalence of subclinical hypothyroidism in obese children or adolescents and association between thyroid hormone and the components of metabolic syndrome. J Paediatr Child Health. 2018 May 16. doi: 10.1111/jpc.13926
Johnston CS, Tjonn SL, Swan PD, White A, Hutchins H, Sears B. Ketogenic low-carbohydrate diets have no metabolic advantage over nonketogenic low-carbohydrate diets. Am J Clin Nutr. 2006 May;83(5):1055-61.
Kang HC, Chung DE, Kim DW, Kim HD. Early- and late-onset complications of the ketogenic diet for intractable epilepsy. Epilepsia. 2004 Sep;45(9):1116-23.
Kang HC, Kim YJ, Kim DW, Kim HD. Efficacy and safety of the ketogenic diet for intractable childhood epilepsy: Korean multicentric experience. Epilepsia. 2005 Feb;46(2):272-9.
Kapadia KB, Bhatt PA, Shah JS. Association between altered thyroid state and insulin resistance. Journal of Pharmacology & Pharmacotherapeutics. 2012;3(2):156-160. doi:10.4103/0976-500X.95517.
Kaplan HS, et al. “A Theory of Human Life History Evolution: Diet, Intelligence, and Longevity.” Evolutionary Anthropology. 2000:9;156-185.
Kim DY, Hao J, Liu R, Turner G, Shi FD, Rho JM. Inflammation-mediated memory dysfunction and effects of a ketogenic diet in a murine model of multiple sclerosis. PLoS One. 2012;7(5):e35476. doi: 10.1371/journal.pone.0035476. Epub 2012 May 2.
Kim JT, Kang HC, Song JE, Lee MJ, Lee YJ, Lee EJ, Lee JS, Kim HD. Catch-up growth after long-term implementation and weaning from ketogenic diet in pediatric epileptic patients. Clin Nutr. 2013 Feb;32(1):98-103. doi: 10.1016/j.clnu.2012.05.019. Epub 2012 Jun 30.
Kinzig KP, Honors MA, Hargrave SL. Insulin sensitivity and glucose tolerance are altered by maintenance on a ketogenic diet. Endocrinology. 2010 Jul;151(7):3105-14. doi: 10.1210/en.2010-0175. Epub 2010 Apr 28.
Klepper J, Leiendecker B, Heussinger N, Lausch E, Bosch F. Severe Hypertriglyceridemia in Glut1D on Ketogenic Diet. Neuropediatrics. 2016 Apr;47(2):132-6. doi: 10.1055/s-0036-1572413. Epub 2016 Feb 22.
Kobayashi J, Saito Y, Taira K, Hikita M, Takahashi K, Bujo H, Morisaki N, Saito Y. Effect of apolipoprotein E3/4 phenotype on postprandial triglycerides and retinyl palmitate metabolism in plasma from hyperlipidemic subjects in Japan. Atherosclerosis. 2001 Feb 15;154(3):539-46.
Kose E, Guzel O, Demir K, Arslan N. Changes of thyroid hormonal status in patients receiving ketogenic diet due to intractable epilepsy. J Pediatr Endocrinol Metab. 2017 Apr 1;30(4):411-416. doi: 10.1515/jpem-2016-0281.
Krikorian R, Shidler MD, Dangelo K, Couch SC, Benoit SC, Clegg DJ. Dietary ketosis enhances memory in mild cognitive impairment. Neurobiol Aging. 2012 Feb;33(2):425.e19-27. doi: 10.1016/j.neurobiolaging.2010.10.006. Epub 2010 Dec 3.
Krogh A. “A study of the diet and metabolism of Eskimos undertaken in 1908 on an expedition to Greenland,” Københaven: C.A. Reitzel, 1914.
Kwiterovich PO Jr, Vining EP, Pyzik P, Skolasky R Jr, Freeman JM. Effect of a high-fat ketogenic diet on plasma levels of lipids, lipoproteins, and apolipoproteins in children. JAMA. 2003 Aug 20;290(7):912-20.
Lager I. The insulin-antagonistic effect of the counterregulatory hormones. J Intern Med Suppl. 1991;735:41-7.
Lambrechts DA, Bovens MJ, de la Parra NM, Hendriksen JG, Aldenkamp AP, Majoie MJ. Ketogenic diet effects on cognition, mood, and psychosocial adjustment in children. Acta Neurol Scand. 2013 Feb;127(2):103-8. doi: 10.1111/j.1600-0404.2012.01686.x. Epub 2012 Jun 12.
Laron Z. Insulin and the brain. Arch Physiol Biochem. 2009 May;115(2):112-6. doi: 10.1080/13813450902949012.
Lin A, Turner Z, Doerrer SC, Stanfield A, Kossoff EH. Complications During Ketogenic Diet Initiation: Prevalence, Treatment, and Influence on Seizure Outcomes. Pediatr Neurol. 2017 Mar;68:35-39. doi: 10.1016/j.pediatrneurol.2017.01.007. Epub 2017 Jan 16.
Lopez-Miranda J1, Ordovas JM, Mata P, Lichtenstein AH, Clevidence B, Judd JT, Schaefer EJ. Effect of apolipoprotein E phenotype on diet-induced lowering of plasma low density lipoprotein cholesterol. J Lipid Res. 1994 Nov;35(11):1965-75.
Lussier DM, Woolf EC, Johnson JL, Brooks KS, Blattman JN, Scheck AC. Enhanced immunity in a mouse model of malignant glioma is mediated by a therapeutic ketogenic diet. BMC Cancer. 2016 May 13;16:310. doi: 10.1186/s12885-016-2337-7.
Mady MA, Kossoff EH, McGregor AL, Wheless JW, Pyzik PL, Freeman JM. The ketogenic diet: adolescents can do it, too. Epilepsia. 2003 Jun;44(6):847-51.
Makris A, Darcey VL, Rosenbaum DL, Komaroff E, Vander Veur SS, Collins BN, Klein S, Wyatt HR, Foster GD. Similar effects on cognitive performance during high- and low-carbohydrate obesity treatment. Nutr Diabetes. 2013 Sep 23;3:e89. doi: 10.1038/nutd.2013.29.
Mavropoulos JC, Yancy WS, Hepburn J, Westman EC. The effects of a low-carbohydrate, ketogenic diet on the polycystic ovary syndrome: a pilot study. Nutr Metab (Lond). 2005 Dec 16;2:35.
McClellan WS and DuBois EF. “Prolonged Meat Diets with a Study of Kidney Function and Ketosis” 1930 The Journal of Biological Chemistry 1930 Jul;87, 651-668.
Melchior JC. From malnutrition to refeeding during anorexia nervosa. Curr Opin Clin Nutr Metab Care. 1998 Nov;1(6):481-5.
Moreno JA, Pérez-Jiménez F, Marín C, Gómez P, Pérez-Martínez P, Moreno R, Bellido C, Fuentes F, López-Miranda J. The effect of dietary fat on LDL size is influenced by apolipoprotein E genotype in healthy subjects. J Nutr. 2004 Oct;134(10):2517-22.
Mosek A, Natour H, Neufeld MY, Shiff Y, Vaisman N. Ketogenic diet treatment in adults with refractory epilepsy: a prospective pilot study. Seizure. 2009 Jan;18(1):30-3. doi: 10.1016/j.seizure.2008.06.001. Epub 2008 Aug 3.
Nakao R, Shimba S, Oishi K. Ketogenic diet induces expression of the muscle circadian gene Slc25a25 via neural pathway that might be involved in muscle thermogenesis. Sci Rep. 2017 Jun 6;7(1):2885. doi: 10.1038/s41598-017-03119-8.
Nam SH, Lee BL, Lee CG, Yu HJ, Joo EY, Lee J, Lee M. The role of ketogenic diet in the treatment of refractory status epilepticus. Epilepsia. 2011 Nov;52(11):e181-4. doi: 10.1111/j.1528-1167.2011.03289.x. Epub 2011 Oct 17.
Ni FF, Li CR, Liao JX, Wang GB, Lin SF, Xia Y, Wen JL. The effects of ketogenic diet on the Th17/Treg cells imbalance in patients with intractable childhood epilepsy. Seizure. 2016 May;38:17-22. doi: 10.1016/j.seizure.2016.03.006. Epub 2016 Mar 22.
Nilsson J, Ericsson M, Joibari MM, Anderson F, Carlsson L, Nilsson SK, Sjödin A, Burén J2. A low-carbohydrate high-fat diet decreases lean mass and impairs cardiac function in pair-fed female C57BL/6J mice. Nutr Metab (Lond). 2016 Nov 15;13:79. doi: 10.1186/s12986-016-0132-8. eCollection 2016.
Paoli A. Ketogenic diet for obesity: friend or foe? Int J Environ Res Public Health. 2014 Feb 19;11(2):2092-107. doi: 10.3390/ijerph110202092.
Ramm-Pettersen A, Stabell KE, Nakken KO, Selmer KK. Does ketogenic diet improve cognitive function in patients with GLUT1-DS? A 6- to 17-month follow-up study. Epilepsy Behav. 2014 Oct;39:111-5. doi: 10.1016/j.yebeh.2014.08.015. Epub 2014 Sep 18.
Rankin JW, Turpyn AD. Low carbohydrate, high fat diet increases C-reactive protein during weight loss. J Am Coll Nutr. 2007 Apr;26(2):163-9.
Rashidian H, Liu YMC, Geraghty MT, Kobayashi J, Donner EJ, Klaassen RJ. Severe Neutropenia and Anemia in a Child With Epilepsy and Copper Deficiency on a Ketogenic Diet. Pediatr Neurol. 2017 Nov;76:93-94. doi: 10.1016/j.pediatrneurol.2017.08.007. Epub 2017 Aug 24.
Reagan LP. Insulin signaling effects on memory and mood. Curr Opin Pharmacol. 2007 Dec;7(6):633-7. Epub 2007 Nov 26.
Rhodes ME, Talluri J, Harney JP, Frye CA. Ketogenic diet decreases circulating concentrations of neuroactive steroids of female rats. Epilepsy Behav. 2005 Sep;7(2):231-9.
Sampath A, Kossoff EH, Furth SL, Pyzik PL, Vining EP. Kidney stones and the ketogenic diet: risk factors and prevention. J Child Neurol. 2007 Apr;22(4):375-8.
Schreck KC, Lwin M, Strowd RE, Henry-Barron BJ, Blakeley JO, Cervenka MC. Effect of ketogenic diets on leukocyte counts in patients with epilepsy. Nutr Neurosci. 2017 Dec 18:1-6. doi: 10.1080/1028415X.2017.1416740. [Epub ahead of print]
Scolnick B. Ketogenic diet and anorexia nervosa. Med Hypotheses. 2017 Nov;109:150-152. doi: 10.1016/j.mehy.2017.10.011. Epub 2017 Oct 13.
Silber TJ. Anorexia nervosa in children and adolescents: diagnosis, treatment and the role of the pediatrician. Minerva Pediatr. 2013 Feb;65(1):1-17.
Simm PJ, Bicknell-Royle J, Lawrie J, Nation J, Draffin K, Stewart KG, Cameron FJ, Scheffer IE, Mackay MT. The effect of the ketogenic diet on the developing skeleton. Epilepsy Res. 2017 Oct;136:62-66. doi: 10.1016/j.eplepsyres.2017.07.014. Epub 2017 Jul 26.
Sirikonda NS, Patten WD, Phillips JR, Mullett CJ. Ketogenic diet: rapid onset of selenium deficiency-induced cardiac decompensation. Pediatr Cardiol. 2012 Jun;33(5):834-8. doi: 10.1007/s00246-012-0219-6. Epub 2012 Feb 25.
Sirven J, Whedon B, Caplan D, Liporace J, Glosser D, O’Dwyer J, Sperling MR. The ketogenic diet for intractable epilepsy in adults: preliminary results. Epilepsia. 1999 Dec;40(12):1721-6.
Spielman LJ, Bahniwal M, Little JP, Walker DG, Klegeris A. Insulin Modulates In Vitro Secretion of Cytokines and Cytotoxins by Human Glial Cells. Curr Alzheimer Res. 2015;12(7):684-93.
Stewart WA, Gordon K, Camfield P. Acute pancreatitis causing death in a child on the ketogenic diet. J Child Neurol. 2001 Sep;16(9):682.
Sunahara KK, Sannomiya P, Martins JO. Briefs on insulin and innate immune response. Cell Physiol Biochem. 2012;29(1-2):1-8. doi: 10.1159/000337579. Epub 2012 Mar 1.
Suo C, Liao J, Lu X, Fang K, Hu Y, Chen L, Cao D, Huang T, Li B, Li C. Efficacy and safety of the ketogenic diet in Chinese children. Seizure. 2013 Apr;22(3):174-8
Swidsinski A, et al. Reduced Mass and Diversity of the Colonic Microbiome in Patients with Multiple Sclerosis and Their Improvement with Ketogenic Diet. Front Microbiol. 2017 Jun 28;8:1141.
Tagliabue A, Ferraris C, Uggeri F, Trentani C, Bertoli S, de Giorgis V, Veggiotti P, Elli M. Short-term impact of a classical ketogenic diet on gut microbiota in GLUT1 Deficiency Syndrome: A 3-month prospective observational study. Clin Nutr ESPEN. 2017 Feb;17:33-37. doi: 10.1016/j.clnesp.2016.11.003. Epub 2016 Dec 18.
Tikkanen MJ, Huttunen JK, Ehnholm C, Pietinen P. Apolipoprotein E4 homozygosity predisposes to serum cholesterol elevation during high fat diet. Arteriosclerosis. 1990 Mar-Apr;10(2):285-8.
Tinsley GM, Willoughby DS. Fat-Free Mass Changes During Ketogenic Diets and the Potential Role of Resistance Training. Int J Sport Nutr Exerc Metab. 2016 Feb;26(1):78-92. doi: 10.1123/ijsnem.2015-0070. Epub 2015 Aug 12.
Tolstoi, E. 1929: “The Effect Of An Exclusive Meat Diet Lasting One Year On The Carbohydrate Tolerance Of Two Normal Men” The Journal of Biological Chemistry 1929 Sept;83, 747-752.
Urbain P, Strom L, Morawski L, Wehrle A, Deibert P, Bertz H. Impact of a 6-week non-energy-restricted ketogenic diet on physical fitness, body composition and biochemical parameters in healthy adults. Nutr Metab (Lond). 2017 Feb 20;14:17. doi: 10.1186/s12986-017-0175-5. eCollection 2017.
Vazquez JA, Adibi SA. Protein sparing during treatment of obesity: ketogenic versus nonketogenic very low calorie diet. Metabolism. 1992 Apr;41(4):406-14.
Vining EP, Pyzik P, McGrogan J, Hladky H, Anand A, Kriegler S, Freeman JM. Growth of children on the ketogenic diet. Dev Med Child Neurol. 2002 Dec;44(12):796-802.
White AM, Johnston CS, Swan PD, Tjonn SL, Sears B. Blood ketones are directly related to fatigue and perceived effort during exercise in overweight adults adhering to low-carbohydrate diets for weight loss: a pilot study. J Am Diet Assoc. 2007 Oct;107(10):1792-6.
Williams S, Basualdo-Hammond C, Curtis R, Schuller R. Growth retardation in children with epilepsy on the ketogenic diet: a retrospective chart review. J Am Diet Assoc. 2002 Mar;102(3):405-7.
Willmott NS, Bryan RA. Case report: scurvy in an epileptic child on a ketogenic diet with oral complications. Eur Arch Paediatr Dent. 2008 Sep;9(3):148-52.
Wilson JM, Lowery RP, Roberts MD, Sharp MH, Joy JM, Shields KA, Partl J, Volek JS, D’Agostino D. The Effects of Ketogenic Dieting on Body Composition, Strength, Power, and Hormonal Profiles in Resistance Training Males. J Strength Cond Res. 2017 Apr 7. doi: 10.1519/JSC.0000000000001935.
Wing RR, Vazquez JA, Ryan CM. Cognitive effects of ketogenic weight-reducing diets. Int J Obes Relat Metab Disord. 1995 Nov;19(11):811-6.
Wroble KA, Trott MN, Schweitzer GG, Rahman RS, Kelly PV, Weiss EP. Low-carbohydrate, ketogenic diet impairs anaerobic exercise performance in exercise-trained women and men: a randomized-sequence crossover trial. J Sports Med Phys Fitness. 2018 Apr 4. doi: 10.23736/S0022-4707.18.08318-4.
Yancy WS Jr, Olsen MK, Guyton JR, Bakst RP, Westman EC. A low-carbohydrate, ketogenic diet versus a low-fat diet to treat obesity and hyperlipidemia: a randomized, controlled trial. Ann Intern Med. 2004 May 18;140(10):769-77.
Zajac A, Poprzecki S, Maszczyk A, Czuba M, Michalczyk M, Zydek G. The effects of a ketogenic diet on exercise metabolism and physical performance in off-road cyclists. Nutrients. 2014 Jun 27;6(7):2493-508. doi: 10.3390/nu6072493.
Zamani GR, et al., 2016: The effects of classic ketogenic diet on serum lipid profile in children with refractory seizures.
Zhao Q, Stafstrom CE, Fu DD, Hu Y, Holmes GL. Detrimental effects of the ketogenic diet on cognitive function in rats. Pediatr Res. 2004 Mar;55(3):498-506. Epub 2004 Jan 7