Although there’s still plenty we don’t know about preventing and treating covid-19, one topic that we should be paying close attention to is the gut microbiome. Research is increasingly suggesting that the collection of critters in our lower GI tract could play a role in the infection process of the SARS-CoV-2 virus and the severity of covid-19 (the disease resulting from the viral infection). Indeed, the gut may offer important clues about how to manage our risk and protect our health!
Table of Contents[Hide][Show]
- A Brief Summary of the Gut Microbiome
- The Microbiome and Viral Infection
- Covid-19 and the Gut: What We Know So Far
- The Gut-Lung Axis
- Age, Microbiota, and COVID-19 Susceptibility
- Gut Microbiota and Comorbidities
- Fermented Foods and Covid-19
- Can We Reduce Covid-19 Risk by Improving Our Gut Microbiome?
- Citations
A Brief Summary of the Gut Microbiome
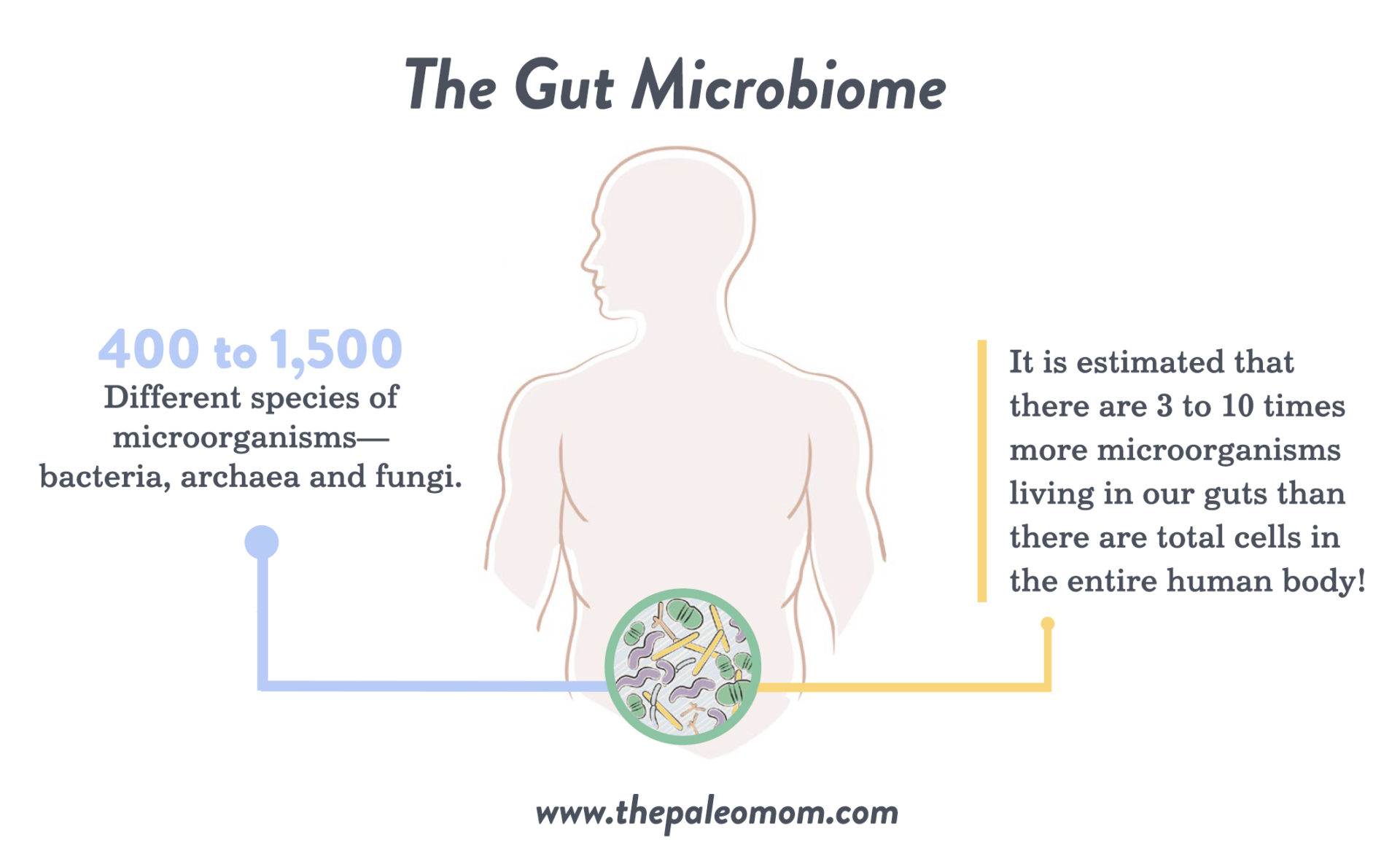
The healthy adult gut is one of the most diverse microbial ecosystems known, home to a vibrant community of microbes that influence nearly all aspects of human biology through their interactions with our bodies. Every person’s gut contains approximately 400 to 1,500 different species of microorganisms—bacteria, archaea and fungi—that are well adapted to survive in the gastrointestinal tract (with about 35,000 species total for all humankind). In fact, it is estimated that there are three to ten times more microorganisms living in our guts than there are total cells in the entire human body! These microorganisms are collectively referred to as our gut microbiota, and the ecosystem as a whole is referred to as our gut microbiome. (See also What Is the Gut Microbiome? And Why Should We Care About It?)
A healthy gut microbiome is working 24/7 to perform many different essential functions that help us to stay healthy. Our gut microbiome acts as a virtual digestive organ, breaking down thousands of food constituents that are incompatible with our own digestive processes, as well as forming important nutrients and impacting their absorption and metabolism. Our gut microbiome regulates and produces many important hormones, including several molecules that double as neurotransmitters. Our gut microbiome contributes to several detoxification pathways. Healthy gut microbiota are critical for the development and maturation of the immune system, helping to maintain the delicate balance required by our immune system by modulating the activities of the various populations of immune cells. And, the gut microbiome directly influences the health and modulates the permeability of the gut barrier—simply restoring a healthy gut microbiome can fix a leaky gut. (See also What Is A Leaky Gut? (And How Can It Cause So Many Health Issues?)).
In fact, every human cell is impacted by the activities of our gut microbes, and we depend on our gut microbiome for health and survival. How does a community of microbes have influence beyond the boundaries of their biological niche, i.e., outside their lovely gut home? Our gut microbes influence our biology through the production of thousands of biologically-active molecules, such as short-chain fatty acids (SCFAs), that are absorbed across the gut barrier and into our bodies. Numerous studies have shown that these microbial metabolites bind with specific receptors embedded within our cell membranes, and in doing so, activate signaling cascades that alter our cellular physiology.
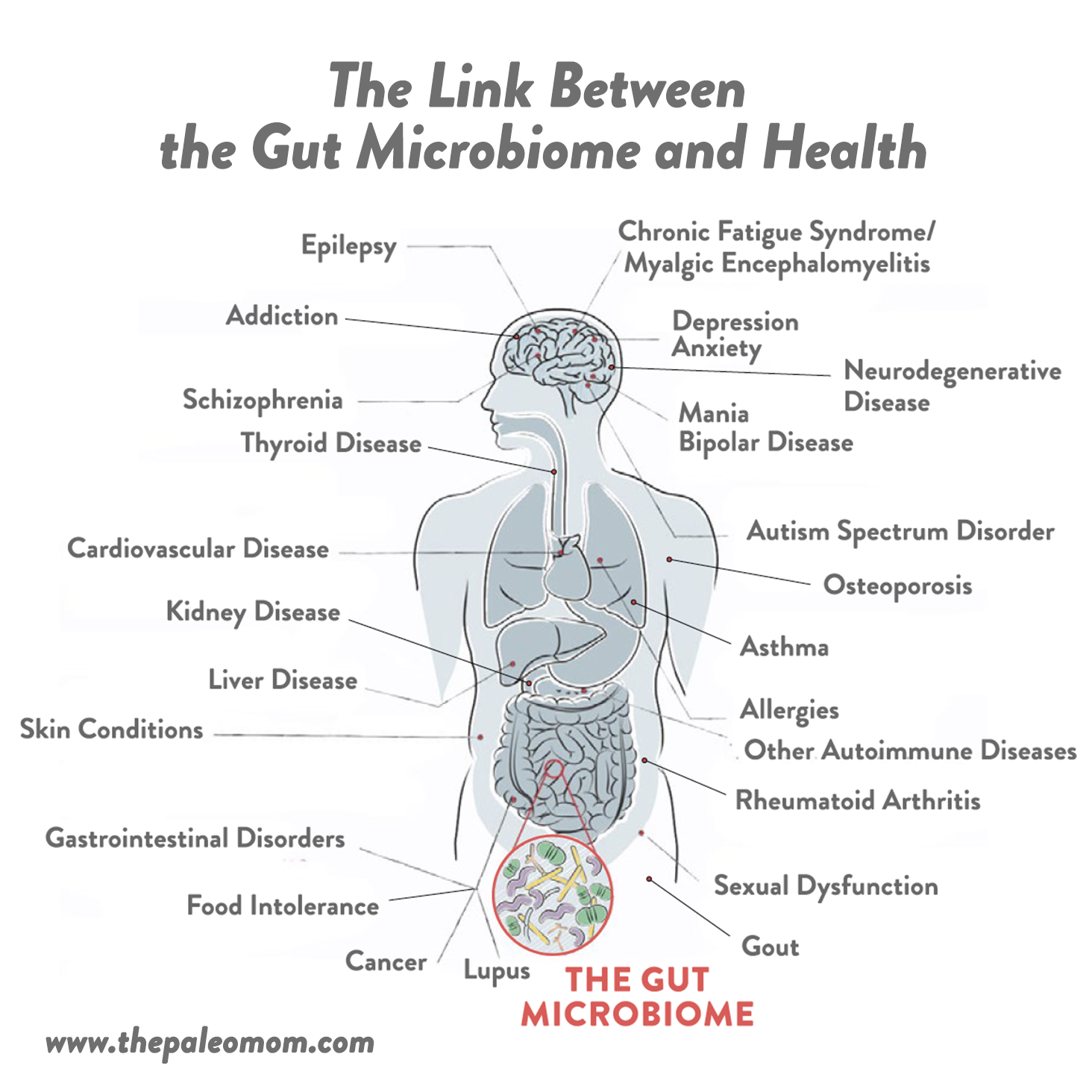
It’s incredible to think that the state of our gut can impact nearly every other aspect of our health, but this is exactly what science continues to show. In fact, at least 90% of all disease can be traced back to the health of the gut and our gut microbiomes. Pathogenic changes in the composition of the gut microbiota is known as gut dysbiosis. This includes too many or too few microorganisms growing in the various segments of the gastrointestinal tract, the wrong kinds of microorganisms, microorganisms in the wrong place, missing important microorganisms, the wrong balance between the different populations of microorganisms, and/or not enough species diversity represented in the community of microorganisms. To date, gut dysbiosis has been linked to: obesity, diabetes, cancer, cardiovascular disease, kidney disease, liver disease, gout, chronic fatigue syndrome/myalgic encephalomyelitis, neurodegenerative disease, schizophrenia, autism spectrum disorder, multiple sclerosis, epilepsy, depression, anxiety, mania, bipolar disease, addiction, lupus, rheumatoid arthritis, multiple sclerosis, thyroid disease, other autoimmune diseases, IBD, IBS, other gastrointestinal diseases, asthma, allergies, food intolerance, skin conditions, osteoporosis, sexual dysfunction, PCOS and…. infection!
The Microbiome and Viral Infection
 There’s a large body of scientific evidence linking viral infections to the microbiome. In general, the gut microbiome can suppress or promote viral infection through a variety of mechanisms, including altering the stability of the virion (the virus before it’s infected a host cell), genetic recombination, driving cell proliferation, suppressing viral replication, stimulating attachment to permissive cells, and influencing local and systemic immune responses. Probiotic species may help protect against viral infections by reducing intestinal permeability, reinforcing the innate immune response in the gut mucosa, and affecting the systemic acquired immune response via anti-inflammatory and regulatory effects. And, some probiotic bacteria (such as Lactobacillus species) are able to directly bind to and inactivate viruses before they attach to host cells, in turn inhibiting infection. Through carbohydrate fermentation and the production of lactic acid, Lactobacillus can also alter pH levels and subsequently inactivate some viruses that are sensitive to changes in acidity. Likewise, short-chain fatty acid (SCFA)-producing bacteria (such as Roseburia, Faecalibacterium prausnitzii, Anaerostipes, and Eubacterium rectale) are important for keeping the lumen replete with butyrate to feed epithelial cells, which produce antiviral compounds. SCFAs produced by fiber fermentation by commensal gut bacteria can also be absorbed by, and act upon, immune cells in ways that reduce the inflammatory component of viral infections like influenza. Likewise, segmented filamentous bacteria can increase ACE2 expression—a transmembrane enzyme that many viruses including SARS-CoV-2 utilize to enter and infect a cell— creating more entry points for viruses like SARS-CoV-2 that bind with these receptors.
There’s a large body of scientific evidence linking viral infections to the microbiome. In general, the gut microbiome can suppress or promote viral infection through a variety of mechanisms, including altering the stability of the virion (the virus before it’s infected a host cell), genetic recombination, driving cell proliferation, suppressing viral replication, stimulating attachment to permissive cells, and influencing local and systemic immune responses. Probiotic species may help protect against viral infections by reducing intestinal permeability, reinforcing the innate immune response in the gut mucosa, and affecting the systemic acquired immune response via anti-inflammatory and regulatory effects. And, some probiotic bacteria (such as Lactobacillus species) are able to directly bind to and inactivate viruses before they attach to host cells, in turn inhibiting infection. Through carbohydrate fermentation and the production of lactic acid, Lactobacillus can also alter pH levels and subsequently inactivate some viruses that are sensitive to changes in acidity. Likewise, short-chain fatty acid (SCFA)-producing bacteria (such as Roseburia, Faecalibacterium prausnitzii, Anaerostipes, and Eubacterium rectale) are important for keeping the lumen replete with butyrate to feed epithelial cells, which produce antiviral compounds. SCFAs produced by fiber fermentation by commensal gut bacteria can also be absorbed by, and act upon, immune cells in ways that reduce the inflammatory component of viral infections like influenza. Likewise, segmented filamentous bacteria can increase ACE2 expression—a transmembrane enzyme that many viruses including SARS-CoV-2 utilize to enter and infect a cell— creating more entry points for viruses like SARS-CoV-2 that bind with these receptors.
A recent meta-analysis of 12 randomized controlled trials (totaling 3720 subjects) found that probiotics were able to reduce the average duration of upper respiratory tract infections, lower the number of infections themselves, and reduce the need for antibiotic administration compared to placebo. Probiotic bacteria like Lactobacillus have immunomodulatory properties and can protect against viral infections by enhancing the cytokine antiviral responses in immune cells and respiratory cells, as well as in the intestinal mucosa. (See also Natural Approaches to Cold & Flu Season (and Covid-19!))
Numerous studies have shown that the gut microbiota are critical for controlling replication of the influenza virus. One study in mice found that altering the gut microbiota with antibiotics increased the severity of influenza infection, while stimulating the microbiota with a high-fiber diet reduced the severity (via enhancing Ly6c-patrolling monocytes). Fecal transfer experiments revealed that dysbiotic gut microbiota reduces the lung’s defenses against influenza-associated pneumonia (more specifically, reduced SCFA production by the bacteria leads to a decrease in the bactericidal activity of alveolar macrophages). This means that an unhealthy gut microbiome may increase susceptibility to bacterial infection while the body is fighting viruses like influenza. What’s more, in mice, oral administration of Lactobacillus brevis was shown to protect against influenza infection by enhancing antiviral IFN-α and augmenting IFV-specific IgA production. Lactobacillus plantarum, meanwhile, was shown to significantly reduce titers of human H1N1 and avian influenza H7N9 in the lungs of mice, while also increasing their survival time after infection with a lethal viral challenge. Lactobacillus paracasei was shown to lower the incidence of influenza A H3N2 infection in mice, while also reducing the infiltration of inflammatory cells into the lungs and leading to faster virus elimination.
The microbiota plays complex roles in a variety of other viral infections, too. Lactobacillus brevis has been shown to interact with components of the herpes simplex virus type 2 to inhibit infection. Lactobacillus gasseri has antiviral activity against respiratory syncytial virus (RSV), with some studies showing that taking this probiotic orally reduces the pulmonary RSV titer, reduces the expression of virus-induced pro-inflammatory mediators in the lungs, and upregulates interferon-stimulated genes. Lactobacillus plantarum was able to reduce inflammation induced by pneumonia virus in rodents. In an experimental rhinovirus infection, Bifidobacterium animalis subspecies lactis BI-04 reduced levels of the pro-inflammatory cytokine IL-6. In human adenovirus type 5, exopolysaccharides produced by Lactobacillus species was shown to exert antiviral activity. Gut microbiota also appears to influence susceptibility to cytomegalovirus (CMV), with Staphylococcus aureus associated with earlier CMV infection in children. And, that’s just the beginning of a long list of systemic viruses impacted by the gut microbiota!
Covid-19 and the Gut: What We Know So Far
Although covid-19 hasn’t been around long enough for us to study its connection with the gut microbiota in great depth, there is a growing number of studies and reviews that have examined the link between the gut microbiota and SARS-CoV-2 infection—including the intestine as an important site of infection, and microbiota patterns among patients with covid-19.
 For starters, let’s look at how this virus can infect us via the gut. SARS-CoV-2 has extremely high binding affinity for angiotensin-converting enzyme II (ACE2), which is how the virus gains entry into host cells: the virus’s spike-like protein binds to the ACE2 like a key fitting into a lock, initiating infection by releasing the fusion machinery that the virus uses to dump its RNA and viral proteins into the target cell, where it then hijacks the cells organelles to produce viral replicas instead of all of the various proteins that the cell needs to survive. (See also Covid-19 FAQ: Do Face Masks Even Work?) Lungs are targeted so strongly during covid-19 because ACE2 is abundantly expressed in lung tissue. But, ACE2 is also expressed in intestinal cells, especially small intestinal and colon epithelial cells (ACE2 serves as a co-receptor for the uptake of nutrients, especially amino acids, and also plays a key role in intestinal homeostasis). This makes the gut a potentially important entry point for SARS-CoV-2. Indeed, studies have shown that about half of covid-19 patients have SARS-CoV-2 RNA detectable in their stools, and the virus has been shown to replicate in enterocytes (intestinal absorptive cells).
For starters, let’s look at how this virus can infect us via the gut. SARS-CoV-2 has extremely high binding affinity for angiotensin-converting enzyme II (ACE2), which is how the virus gains entry into host cells: the virus’s spike-like protein binds to the ACE2 like a key fitting into a lock, initiating infection by releasing the fusion machinery that the virus uses to dump its RNA and viral proteins into the target cell, where it then hijacks the cells organelles to produce viral replicas instead of all of the various proteins that the cell needs to survive. (See also Covid-19 FAQ: Do Face Masks Even Work?) Lungs are targeted so strongly during covid-19 because ACE2 is abundantly expressed in lung tissue. But, ACE2 is also expressed in intestinal cells, especially small intestinal and colon epithelial cells (ACE2 serves as a co-receptor for the uptake of nutrients, especially amino acids, and also plays a key role in intestinal homeostasis). This makes the gut a potentially important entry point for SARS-CoV-2. Indeed, studies have shown that about half of covid-19 patients have SARS-CoV-2 RNA detectable in their stools, and the virus has been shown to replicate in enterocytes (intestinal absorptive cells).
Not surprisingly, a significant number of covid-19 patients (10–20%, according to meta-analyses conducted so far) experience GI symptoms like diarrhea, vomiting, and nausea; this may be due to: reduced intestinal ACE2 expression (SARS-CoV-2 infection decreases ACE2 expression in the lungs and likely does so in the gut, too; see also TWV Podcast Episode 412: Covid-19 FAQ, Part 3); changes in oxygen levels; or, effects on the central nervous system and gut-brain axis (for example, an immune response that induces diarrhea or stimulates the vagus nerve to cause vomiting). Similarly, viral replication within the GI tract could exponentially increase the SARS-CoV-2 load in the gut mucosa, leading to a loss of barrier integrity, imbalance of residence microbes and metabolites (like SCFAs), and subsequent alterations in the immune system—including high cytokine production. A study published this week in Science showed an abundance of gut bacterial DNA and endotoxin in the blood of covid-19 patients, correlating with disease severity.
Along with these effects on the gut, covid-19 infection may alter the gut microbiota itself. Decreases in ACE2 are known to reduce the production of certain antimicrobial peptides that help modulate the microbiota composition, and studies of covid-19 patients specifically have shown that infection is associated with significantly lower bacterial diversity and abundance (including lower relative abundance of butyrate-producing bacteria from the probiotics Ruminococcaceae and Lachnospiraceae, Alistipes onderdonkii, Roseburia, and Faecalibacterium prausnitzii), along with a greater abundance of opportunistic pathogens such as Clostridium hathewayi, Rothia, Veillonella, Actinomyces viscosus, Bacteroides nordii, and Streptococcus. Across multiple studies, an overabundance of Prevotella has been found in infected patients; a study in China found low levels of the essential probiotics Lactobacillus and Bifidobacterium. (Some of these bacteria are also discussed in Paleo, Resistant Starch, and TMAO: New Study Warning Worth Heeding and The Link Between Meat and Cancer.)

A small study of covid-19 patients from Hong Kong found that the abundance of Coprobacillus, Clostridium ramosum, and Clostridium hathewayi positively correlated with disease severity, while the probiotic Faecalibacterium prausnitzii negatively correlated with disease severity—possibly due to the viral infection setting the stage for secondary bacterial infection. A pattern of depletion of symbiotic bacteria and enrichment with opportunistic pathogens persisted even after recovery from SARS-CoV-2 (demonstrated by negative results from throat swab and stool). Likewise, a number of bacteria known to downregulate intestinal ACE2 expression in rodents (including Bacteroides dorei, Bacteroides thetaiotaomicron, Bacteroides massiliensis, and Bacteroides ovatus) inversely correlated with the SARS-CoV-2 load in patients’ fecal samples. Fascinatingly, when covid-19 patients were compared with healthy controls, covid-19 infection demonstrated the greatest impact on the gut microbiome compared to other host factors like antibiotics, hyperlipidemia, age, gender, or pneumonia.
Studies of covid-19 also show that infection alters the gut-blood barrier and allows greater entry of systemic endotoxins, bacteria, and microbial metabolites (yes, leaky gut, see also What Is A Leaky Gut? (And How Can It Cause So Many Health Issues?)). In turn, this can affect the body’s initial response to infection and lead to septic shock, cytokine storms, and multisystem dysfunction that can potentially be fatal. (See also Natural Approaches to Cold & Flu Season (and Covid-19!).)
It’s important to note that these gut-related changes appear to be a consequence of SARS-CoV-2 infection, rather than being baseline features that predispose people to getting infected in the first place. It remains unknown how much these gut microbiome changes contribute to the long-term syndrome-like symptoms faced by many covid-19 patients, or if permanent alterations of the gut microbiome due to SARS-CoV-2 could set the stage for future chronic illness.
There are also some clues that the state of the gut microbiota could make someone more or less susceptible to this virus!
A recent Chinese study (currently pre-print) investigated whether certain proteomic biomarkers predicting severe covid-19 progression among infected individuals could also be used to explain disease susceptibility among people who weren’t yet infected—and whether the gut microbiota was involved in regulating those biomarkers. Using a pool of over 300 healthy individuals with data spanning a three years, the researchers identified core gut microbiota features that could predict the proteomic biomarkers and potentially indicate someone’s susceptibility to covid-19: Bacteroides, Streptococcus, Lactobacillus, Ruminococcaceae, Lachnospiraceae, and Clostridiales. In fact, Pearson correlation analysis showed a highly significant 0.59 coefficient between the bacteria-predicted blood proteomic risk score (PRS) and the actual proteomic risk score of the participants—making gut bacteria a better predictor than any other demographic characteristic or laboratory test! The relationship between proteomic risk score and infection was most significant among older individuals. As far as inflammation went, Bacteroides, Streptococcus, and Clostridiales were negatively associated with most of the inflammatory cytokines tested (IL-1β, IL-2, IL-4, IL-6, IL-8, IL-10, IL-12p70, IL-13, TNF-α and IFN-γ), while Ruminococcus, Blautia, and Lactobacillus were positively associated with most of these cytokines. The researchers proposed that bacterial metabolites identified in the feces (mostly those associated with amino acid biosynthesis pathways) likely played a key role in mediating the effects of the gut microbiota upon inflammation and metabolism in the host. And most importantly, among these healthy individuals, alterations in the gut microbiota composition preceded changes in the proteomic risk score, pointing to the gut microbiota as the cause of these risk-raising changes (rather than the biomarker changes causing gut dysbiosis). Overall, this fascinating study suggests that certain disruptions in the gut microbiota could predispose individuals to an inflammatory status that increases covid-19 susceptibility and severity.
Although less studied, evidence also suggests that the gut microbiota could play a role in the cytokine storms associated with SARS-CoV-2 infection. Cytokine storms are an over-reactive immune response to infection, in which a disproportionate amount of pro-inflammatory cytokines (small proteins that act as immune messengers, like IL-1β, IL-6, and TNF-α) are released into the bloodstream too quickly and can cause hyper-inflammation, damage cells, and lead to multisystem organ failure and death. Metabolic processes by the gut microbiota strongly influence cytokine production, and the gut microbiota composition may contribute to cytokine reactions seen in severe covid-19 cases.
The Gut-Lung Axis
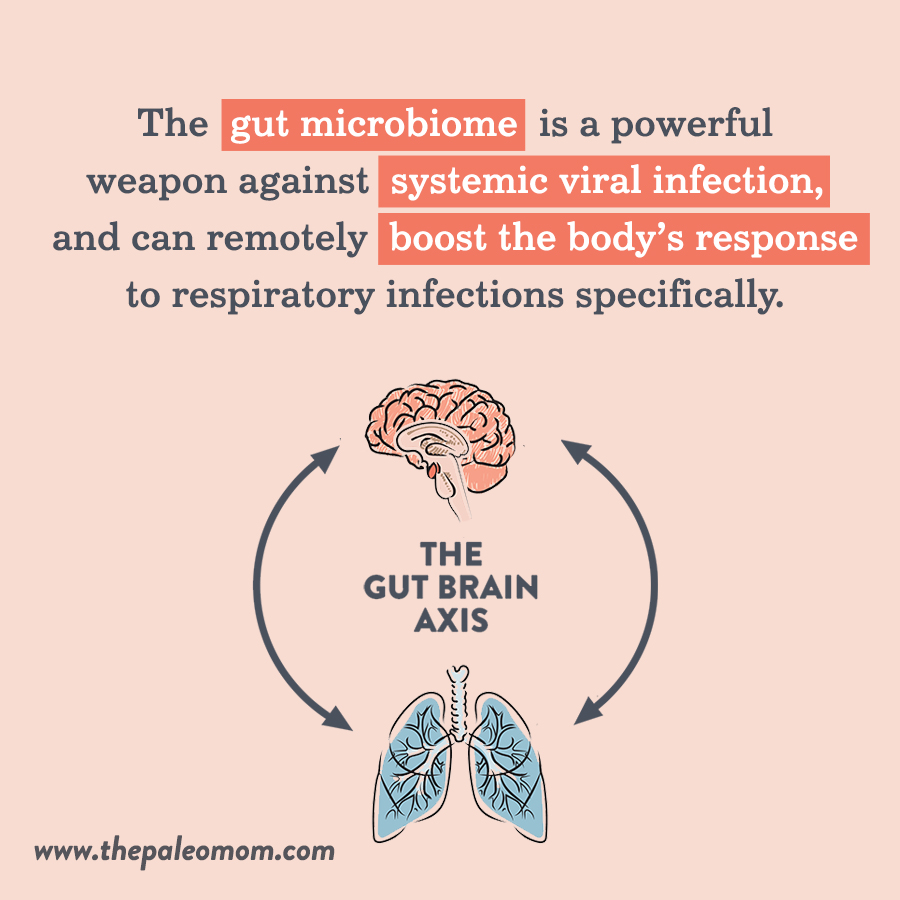 The gut microbiome interacts with a number of other organs and body systems beyond the GI tract, and the lungs are no exception! (See also How Stress Undermines Health and How Chronic Stress Leads to Hormone Imbalance). The gut-lung axis describes how the composition of the gut microbiota influences susceptibility to lung disease, including viral infections (and as we’ve seen, viral infection can likewise alter the composition of the gut microbiota).
The gut microbiome interacts with a number of other organs and body systems beyond the GI tract, and the lungs are no exception! (See also How Stress Undermines Health and How Chronic Stress Leads to Hormone Imbalance). The gut-lung axis describes how the composition of the gut microbiota influences susceptibility to lung disease, including viral infections (and as we’ve seen, viral infection can likewise alter the composition of the gut microbiota).
In general, the gut microbiome is a powerful weapon against systemic viral infection, and can remotely boost the body’s response to respiratory infections specifically. The gut microbiota can enhance CD8+ T cell effector function (such as cytotoxic T cells, which promote viral clearance by inducing apoptosis [programmed cell death] of infected cells), and components of the bacterial cell wall, as well as bacterial metabolites like desaminotyrosine (produced by the commensal bacteria Clostridium orbiscendens), support the production of inflammasome-dependent cytokines and type I interferons in lung cells. In mice, segmented filamentous bacteria in the gut have been shown to stimulate pulmonary type 17 helper T cell (Th17) responses, along with protecting against S. pneumonia infection and death; mouse studies have also shown that Bifidobacteria species protect against both viral and bacterial lung infection. So, all the way from our lower GI tract, microbes have a say in what happens in our lungs!
Even beyond viral infections, the gut microbiota have shown important links with maintaining healthy lung function and protecting against airway diseases. For example, SCFAs produced by fiber-fermenting bacteria appear to suppress airway inflammation, leading to improvements in asthma symptoms. One study in mice found that a high-fiber diet increased the production of the SCFA acetate in the gut, in turn enhancing the number and function of regulatory T cells (acetate increases acetylation at the Foxp3 promoter) and suppressing the animals’ allergic airway disease (the equivalent to human asthma). A separate study found that high-fiber feeding conferred protection against food allergies by enhancing the activity of retinal dehydrogenase in CD103(+) DC. Another rodent study found that increasing the fermentable fiber content of the animals’ diet altered their gut and lung microbiomes, leading to higher levels of circulating short-chain fatty acids, and in turn protecting against allergic inflammation in the lungs (in contrast to a low-fiber diet, which increased allergic lung inflammation).
Can we just take yet another moment to say “yay fiber!” Learn more in What Is Fiber and Why Is it Good?, The Many Types of Fiber, Soluble vs. Insoluble Fiber, Fiber, Cholesterol and Bile Salts, Busting the Abrasive Insoluble Fiber Myth, New Science Suggests Fiber Improves Sleep Quality!, Why Fruit is a Good Source of Carbohydrates, Why Root Veggies Are Great for the Gut Microbiome, The Health Benefits of Citrus Fruits, The Health Benefits of Apples, and The Importance of Vegetables.
Age, Microbiota, and COVID-19 Susceptibility
It’s well established that even with high viral loads, children and young adults are much less likely to develop symptomatic covid-19 than older adults. In fact, older age is a major risk factor for severe or fatal covid-19 cases. Could the gut microbiome play a role here, too?
It’s a solid possibility! For example, older individuals tend to display chronic low-grade inflammation and reduced gut barrier integrity (leaky gut), both of which can exacerbate the problems associated with SARS-CoV-2 infection. Likewise, throughout life, the gut microbiota goes through predictable shifts in composition, and by the age of about 60, the average gut microbiome exhibits a drop in diversity and Lactobacillus and Bifidobacterium abundance—potentially translating to greater susceptibility to systemic viral infections like SARS-CoV-2, due to the role of the gut microbiota in immunity (and the specific role of Lactobacillus species in binding to viruses). Indeed, research has shown that age-associated changes in the immune system and inflammation are strongly modulated by changes in the gut microbiota, and can collectively create an over-reactive inflammatory phenotype that predisposes to cytokine storms and other damaging activity for the vital organ systems. As further evidence, a recent study showed that older individuals exhibit a significant association between certain covid-19-related blood proteomic markers and inflammation, and may be particularly susceptible to the cytokine storm associated with more severe cases.
Gut Microbiota and Comorbidities
By now, we have plenty of data showing that certain comorbidities increase the risk of severe or fatal covid-19 infection—particularly diabetes, high blood pressure, and obesity, but also kidney disease, heart disease, COPD, and cancer. (In fact, an exception to the trend of younger people being more protected against severe covid-19 is when one of these comorbidities is involved: regardless of age, someone with diabetes or obesity is at risk of more severe disease than someone without these conditions.) And, it just so happens that these conditions are consistently associated with alterations in the gut microbiota. Whether these comorbidities are such high risk factors for covid-19 due to a role of gut dysbiosis is certainly worth exploring!
One interesting pattern is that these chronic diseases tend to correlate with a lower abundance of Bacteroides species—some of which are reported to suppress ACE2 expression in the colon and calibrate the immune response of the host. That means that some of the microbiota alterations coinciding with these conditions could reduce the body’s ability to fight off SARS-CoV-2 while also providing more entry sites for the virus in the intestine. In addition, infection with SARS-CoV-2 may be particularly dangerous in the presence of comorbidities due to amplifying systemic pathways through gut-related avenues—such as ACE2 disruption, alterations in microbial profiles, intestinal inflammation, and loss of gut barrier integrity.
On a more specific level, each co-morbidity has microbiota signatures that could lower systemic immunity and/or increase ACE2 expression—in both cases, granting entry for SARS-CoV-2 and supporting a higher viral load. For example, microbiota profiles associated with obesity include a lower proportion of Bacteroidetes (while these bacteria tend to be higher among lean individuals), theoretically making the obesity-associated gut microbiota less protective against SARS-CoV-2 infection. Cardiovascular disease is associated with higher levels of endotoxin, a highly inflammatory component of the outer layer of Gram-negative bacteria; along with its role in atherosclerosis (endotoxin is involved in both the initiation and progression of atherosclerosis through pro-coagulant activity, endothelial cell injury, monocyte recruitment, and the transformation of macrophages into foam cells), endotoxin can contribute to systemic inflammation that may increase the risk of a cytokine storm associated with COVID-19. And, many types of cancer are associated with microbiomes low in SCFA-producing bacteria, which could reduce the availability of butyrate for epithelial cells and potentially lessen the body’s defenses against viral infection.
Recent research has suggested an important role of the gut microbiome in chronic kidney disease—another co-morbidity that increases the risk of severe covid-19. Patients with kidney disease tend to have microbiomes characterized by higher production of uremic toxins and low production of short-chain fatty acids (in turn, potentially reducing immune function). Gut dysbiosis can lead to dysfunction of the intestinal barrier and the translocation of bacterial DNA and gut-derived toxins into the bloodstream, eventually triggering a state of systemic inflammation—another predisposing factor for more severe covid-19. Patients with chronic kidney disease also have higher proportions of opportunistic pathogens from gamma-Proteobacteria, and have lower levels of beneficial strains of Roseburia, Coprococcus, Bifidobacteria, Lactobacilli, and Ruminococcaceae, raising the risk of secondary infections from respiratory viruses. Collectively, these features would be expected to make kidney disease patients much more susceptible to SARS-CoV-2 infection due to the impact on intestinal and systemic immunity. In addition, dietary treatments for kidney disease can also contribute to an altered gut microbiome that raises severe covid-19 risk. In order to reduce complications like hyperkalemia, patients with chronic kidney disease are prescribed diets low in fiber, protein, and symbiotic organisms—again causing shifts in the microbiota that reduce production of SCFAs and possibly increase the risk of viral infection and replication. (See also The Paleo Diet for Kidney Disease and The Paleo Diet for Gout).
Fermented Foods and Covid-19
 Very recently, a review paper from Europe noted that across countries and regions within countries, cabbage and fermented vegetable consumption is associated with lower covid-19 death rates (as measured by the Comprehensive European Food Consumption Database tracking consumption of fermented vegetables, fermented milk, yogurt, and pickled/marinated vegetables). Out of all the variables examined, fermented vegetables were the only thing that reached statistical significance with national covid-19 death rate. In fact, for each gram per day increase in fermented vegetable intake per capita, covid-19 mortality risk dropped by 35.4%! (Meanwhile, a separate analysis of various vegetables found that only cabbage and cucumber were significantly associated with reduced covid-19 mortality.) See also The Health Benefits of Fermented Foods and Natural Approaches to Cold & Flu Season (and Covid-19!).
Very recently, a review paper from Europe noted that across countries and regions within countries, cabbage and fermented vegetable consumption is associated with lower covid-19 death rates (as measured by the Comprehensive European Food Consumption Database tracking consumption of fermented vegetables, fermented milk, yogurt, and pickled/marinated vegetables). Out of all the variables examined, fermented vegetables were the only thing that reached statistical significance with national covid-19 death rate. In fact, for each gram per day increase in fermented vegetable intake per capita, covid-19 mortality risk dropped by 35.4%! (Meanwhile, a separate analysis of various vegetables found that only cabbage and cucumber were significantly associated with reduced covid-19 mortality.) See also The Health Benefits of Fermented Foods and Natural Approaches to Cold & Flu Season (and Covid-19!).
Although we should take this correlation with a giant grain of salt (given the numerous potential confounding factors!), there’s actually some mechanistic plausibility for cabbage and fermented foods being protective during SARS-CoV-2 infection. Sulforaphane from cabbage and Lactobacillus (found in lacto-fermented vegetables) are among the most active natural activators of nuclear factor erythroid 2–related factor 2 (Nrf2), which can block the angiotensin II receptor type 1 (AT1R) axis—an axis associated with oxidative stress, and which becomes enhanced as SARS-CoV-2 binds and downregulates ACE2. Nrf2 has anti-fibrotic effects on the lungs, protects against endothelial damage and lung injury, and protects against acute respiratory distress syndrome—all hallmarks of severe covid-19. It’s not a stretch to speculate that Lactobacillus-containing foods could be protective through this avenue.
Can We Reduce Covid-19 Risk by Improving Our Gut Microbiome?
Don’t rush out to the probiotic aisle in your local grocery store just yet (and if you do, wear a mask, see Covid-19 FAQ: Do Face Masks Even Work?). It’s too early to make any declarative statements about gut-microbiota-modifying strategies, such as taking particular strains of probiotics, impacting susceptibility to or the course of covid-19. We simply don’t have the research yet. But, based on known mechanisms along with studies currently available on covid-19, we can say that improving the state of our gut health certainly won’t hurt, and may improve a number of immune parameters—including ones directly involved with viral infection.
 One thing we do know for sure though: Supporting a healthy and diverse gut microbial community is a prerequisite for our good health. In fact, in my six-year-long deep dive into gut microbiome research to write The Gut Health Guidebook, I have yet to find a chronic illness that is not linked to a dysfunctional gut microbiome. Our health really is rooted in our guts.
One thing we do know for sure though: Supporting a healthy and diverse gut microbial community is a prerequisite for our good health. In fact, in my six-year-long deep dive into gut microbiome research to write The Gut Health Guidebook, I have yet to find a chronic illness that is not linked to a dysfunctional gut microbiome. Our health really is rooted in our guts.
In my research, I became obsessed with understanding how healthy food choices benefit us, not directly, but instead via improving the gut microbiome. Diet is the single biggest influence on microbiota composition. In fact, diet is directly responsible for more than 60% of the variation in bacterial species in the gut, with probiotic exposure, genetics, gender, age, lifestyle, hormones, drugs, supplements, toxin and environmental exposures collectively responsible for the remaining 40%. As I gathered information, I was very intentional to follow the science wherever it led, rather than cherrypick only those studies that rationalized the standard Paleo or AIP templates. So, in my new e-book The Gut Health Guidebook, I build a new diet for general health from the ground up based on optimizing the gut microbiome.
There’s a really immense amount of information in The Gut Health Guidebook, but it all boils down to 20 keys to gut health. Here’s the cliffsnotes: To best support a healthy gut microbiome, eat a nutrient-dense and varied diet that is moderate-fat and moderate-carb and that includes plenty of veggies, fruit, mushrooms, and seafood, rounded out with nuts, seeds, grass-fed meats, fermented foods, and phytochemical-rich foods like herbs, tea, coffee, cacao and extra virgin olive oil. Lifestyle factors are also essential, like getting enough sleep on a consistent schedule, entrenching a solid circadian rhythm, eating distinct meals instead of grazing, fasting overnight (12-14 hours, not IFing), living an active lifestyle, and managing stress. It’s also super important to optimize vitamin D levels. There are a handful of foods that are traditionally excluded on the Paleo diet that actually are a boon to our gut microbiomes, including A2 dairy (like goat, sheep or camel), most legumes (not soy or peanuts), pseudograins, corn, rice, and gluten-free oats; however, none of these latter foods are fundamental for a healthy gut microbiome in the same way that mushrooms, seafood, and individual families of vegetables and fruit are.
Of course, you can super nerd out on all the details behind all 20 keys to gut health, as well as how the aforementioned non-Paleo foods fit into the picture, in The Gut Health Guidebook (and don’t forget to check out The Gut Health Collection because the companion e-cookbook, which includes 180+ recipes centered on 61 gut health superfoods, is coming is September!).
Citations
Antunes A, et al. “Potential contribution of beneficial microbes to face the COVID-19 pandemic.” Food Res Int. 2020 Oct; 136: 109577.
Biliavska L, et al. “Antiviral Activity of Exopolysaccharides Produced by Lactic Acid Bacteria of the Genera Pediococcus, Leuconostoc and Lactobacillus against Human Adenovirus Type 5.” Medicina (Kaunas). 2019 Sep; 55(9): 519.
Bousquet J, et al. “Cabbage and fermented vegetables: from death rate heterogeneity in countries to candidates for mitigation strategies of severe COVID‐19.” Allergy. Accepted Author Manuscript. 6 Aug 2020. doi:10.1111/all.14549
Budden KF, et al. “Emerging pathogenic links between microbiota and the gut-lung axis.” Nat Rev Microbiol. 2017 Jan;15(1):55-63. doi: 10.1038/nrmicro.2016.142. Epub 2016 Oct 3.
Carvalho-Queiroz C, et al. “Associations between EBV and CMV Seropositivity, Early Exposures, and Gut Microbiota in a Prospective Birth Cohort: A 10-Year Follow-up.” Front Pediatr. 2016; 4: 93.
Gou W, et al. “Gut microbiota may underlie the predisposition of healthy individuals to COVID-19.” MedRxiv. doi: https://doi.org
He LH, et al. “Intestinal Flora as a Potential Strategy to Fight SARS-CoV-2 Infection.” Front Microbiol. 2020; 11: 1388.
Infusino F, et al. “Diet Supplementation, Probiotics, and Nutraceuticals in SARS-CoV-2 Infection: A Scoping Review.” Nutrients. 2020, 12(6), 1718; https://doi.org/10.3390/nu12061718
Kalantar-Zadeh K, et al. “Considering the Effects of Microbiome and Diet on SARS-CoV-2 Infection: Nanotechnology Roles.” ACS Nano. 2020 May 1: acsnano.0c03402.
Li, N et al. “The Commensal Microbiota and Viral Infection: A Comprehensive Review.” Front Immunol. 2019; 10: 1551.
Mendes V, et al. “Mechanisms by Which the Gut Microbiota Influences Cytokine Production and Modulates Host Inflammatory Responses.” J Interferon Cytokine Res. 2019 Jul;39(7):393-409. doi: 10.1089/jir.2019.0011. Epub 2019 Apr 23.
Tan J, et al. “Dietary Fiber and Bacterial SCFA Enhance Oral Tolerance and Protect against Food Allergy through Diverse Cellular Pathways.” Cell Rep. 2016 Jun 21;15(12):2809-24. doi: 10.1016/j.celrep.2016.05.047.
Trompette A, et al. “Dietary Fiber Confers Protection against Flu by Shaping Ly6c – Patrolling Monocyte Hematopoiesis and CD8 + T Cell Metabolism.” Immunity. 2018 May 15;48(5):992-1005.e8. doi: 10.1016/j.immuni.2018.04.022.
Trottein F & Sokol H. “Potential Causes and Consequences of Gastrointestinal Disorders during a SARS-CoV-2 Infection.” Cell Rep. 2020 Jul 21; 32(3): 107915.
Viana SD, et al. “ACE2 imbalance as a key player for the poor outcomes in COVID-19 patients with age-related comorbidities – Role of gut microbiota dysbiosis.” Ageing Res Rev. 2020 Sep; 62: 101123.
Zuo T, et al. “Alterations in Gut Microbiota of Patients With COVID-19 During Time of Hospitalization.” Gastroenterology. 2020 May 20. doi: 10.1053/j.gastro.2020.05.048












