 The collagen supplement market is booming. There’s gelatin, collagen protein, collagen peptides, hydrolyzed collagen, collagen hydrolysate, marine collagen, multi-collagen, bone broth collagen, and bone broth protein, each packaged and sold in an ever increasing collection of products, from protein powders to protein bars, cookies, beverages, coffee creamers, capsules, gummies, and more. While American consumers spent about $50 million on collagen supplements in 2014, we’re expected to spend upwards of $293 million on collagen supplements in 2020. According to the market research firm Nutrition Business Journal, the global market is projected to reach $7.5 billion by 2027.
The collagen supplement market is booming. There’s gelatin, collagen protein, collagen peptides, hydrolyzed collagen, collagen hydrolysate, marine collagen, multi-collagen, bone broth collagen, and bone broth protein, each packaged and sold in an ever increasing collection of products, from protein powders to protein bars, cookies, beverages, coffee creamers, capsules, gummies, and more. While American consumers spent about $50 million on collagen supplements in 2014, we’re expected to spend upwards of $293 million on collagen supplements in 2020. According to the market research firm Nutrition Business Journal, the global market is projected to reach $7.5 billion by 2027.
Table of Contents[Hide][Show]
As the market saturates with collagen-based products and manufacturers vie for your dollar (not to mention brand loyalty) with compelling claims, it’s important to be an informed consumer. Not all collagen supplements are created equal.
So, let’s dissect the three main collagen supplements that form the basis of the full range of products on the market: gelatin, collagen peptides (also called hydrolyzed collagen, collagen hydrolysate, gelatin hydrolysate, or collagen protein), and bone broth protein. First, we’ll dive into what exactly collagen is and the biological effects of increasing intake whether from supplements or food sources. Then, we’ll compare how the various supplemental forms are extracted and processed, their digestibility, the relevance of the source material (c.f. bovine hide collagen peptides, marine collagen and multi-collagen) and what to look for on (and off!) labels.
First, What Is Collagen?
 Let’s begin with what collagen actually is so we can understand why we stand to benefit from consuming it.
Let’s begin with what collagen actually is so we can understand why we stand to benefit from consuming it.
Collagen is the most abundant protein in our bodies, accounting for approximately 30% of all our proteins, and is our dominant structural protein, including being a main building block of connective and interstitial tissues, bone, cartilage, ligaments, tendons, and skin. It’s also abundant in muscles, blood vessels, corneas, and teeth. The word collagen is derived from the Greek “kólla,” which means glue, although collagen functionally acts both as a glue—holding our cells, tissues and organs together—as well as a structural scaffold.
I want to dig into the forms and functions of collagen in even more detail, since it’s relevant to some collagen supplement claims. Head’s up: this is about to get very sciency, so feel free to skip to the next section. TL;DR? Collagen is a crazy important protein in our bodies and it does a bunch of awesome things. We don’t make it as well when we age or are stressed or inflamed, and that’s not cool.
There are 29 currently-identified genetically-distinct types of collagen (given roman numerals in order of discovery), encoded by at least 46 genes, and categorized by their quaternary structures and architecture, i.e., how collagen molecules link together in different ways in order to perform various functions. And yet, all collagens are based on a characteristic triple helix tertiary structure, which is formed by three polypeptide chains (long chains of amino acids, called α-chains, each predominantly composed of a repeating sequence of three amino acids, and varying in length from about 600 amino acids to over 3000 amino acids long) that tightly twist around each other. About a third of the amino acids in collagen are glycine because glycine is always the first amino acid in the repeating sequence of three amino acids that forms the α-chains—the hydrogen bonds between adjacent glycine molecules is what gives the triple helix its strength and stability. Proline and hydroxyproline are commonly one of the other two amino acids in the repeating sequence.
The collagen triple helix, also called procollagen, undergoes post-translational modifications to become a basic collagen molecule (also called tropocollagen). Collagen molecules spontaneously self-assemble into a diversity of larger structures, influenced not only by the constituent α-chains (the combination of different α-chains determines which of the 29 types of collagen it is) but also by other matrix molecules (such as elastin, keratin and proteoglycans) as well as adjacent cellular elements.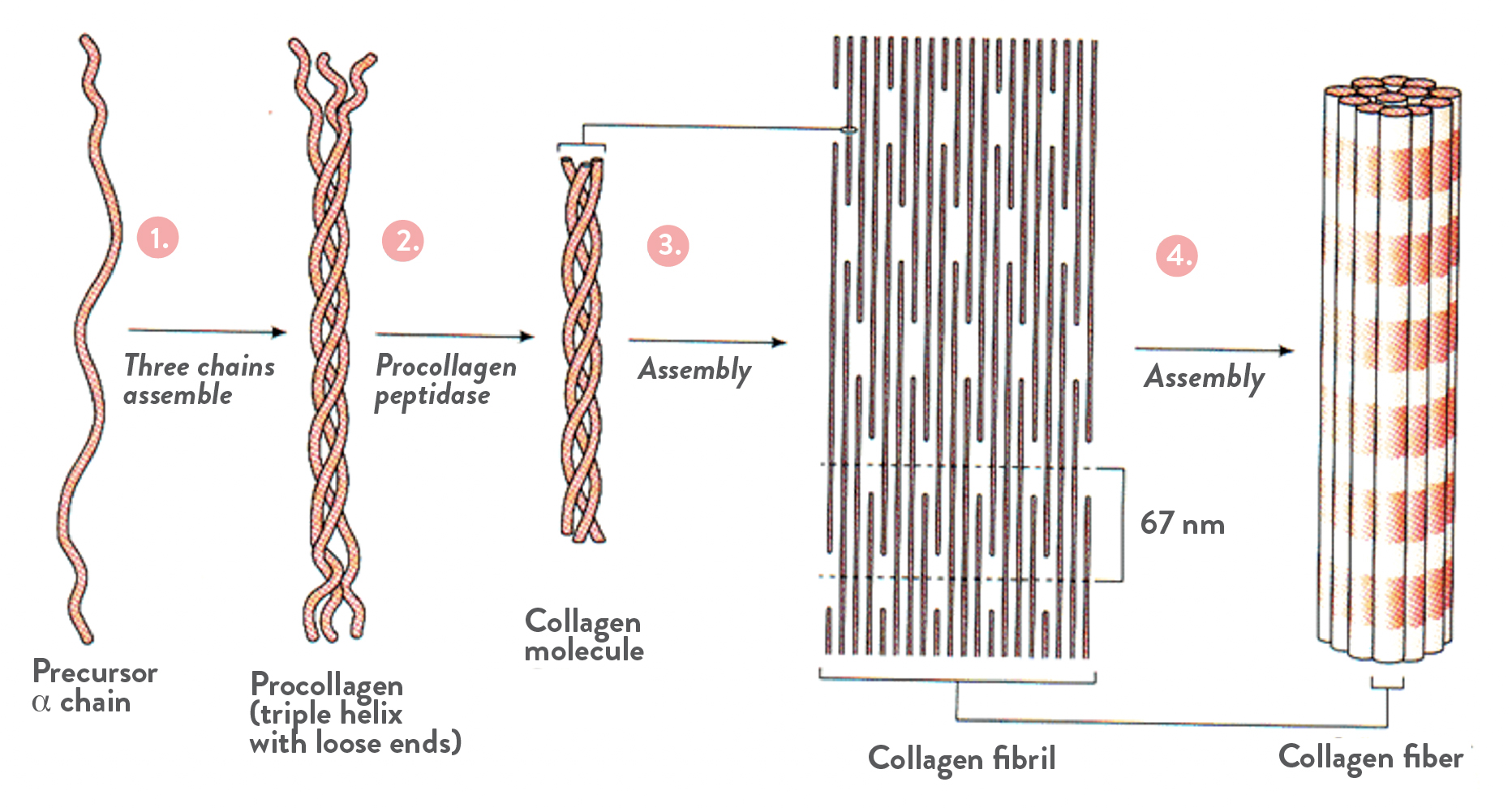
The most common and abundant type of collagen in our bodies—type I collagen, which accounts for about 90% of the collagen in our bodies—is categorized as a fibrillar collagen because the collagen molecules align to form fibrils, which then self-assemble to form collagen fibers. It’s very analogous to how a rope (=collagen fiber) is made of several twisted strands (=collagen fibrils), each made of several twisted yarns (=collagen triple helix), each made of spun fibers (=α-chains). This assembly provides type I collagen fibers with amazing strength and flexibility, the biomechanical properties that provide tensile strength and shock-absorption to bones, load-bearing to tendons and ligaments, elasticity to skin, and determine the tensile strength of the extracellular matrix. Type I collagen is found in skin, tendon, ligament, cornea, fibrocartilage, organ capsules, dura mater of brain, spinal cord, and skeletal muscle perimysium, and is the main organic component of bone.
While about 90% of our collagen is type I collagen, most of the remainder are types II, III, IV, V and IX. Interestingly, most tissues tend to include multiple collagen types with very small amounts of secondary collagen types serving as important regulators of collagen assembly and modifiers of biomechanical properties. In fact, there’s a role for all 29 collagen types in our bodies, even if the amount of most of those collagen types is incredibly small, especially relative to type I collagen.
Type II collagen is also a fibrillar collagen and makes up about 50% of all of the proteins in our cartilage. In fact, type II collagen makes up 85% of the collagen nucleus pulposus of spinal discs, imparting compressibility and shock-absorption, and about 90% of the collagen in articular (hyaline and elastic) cartilage, imparting strength and compressibility.
Type III collagen is also fibrillar and tends to work alongside type I collagen (as a copolymer) in skin, tendons, ligaments, vascular walls, skeletal muscle endomysium and epimysium, and other tissues, and is a major component of the extracellular matrix, providing compliance in these tissues. The reticular fibers in reticular tissue—fine fibrous connective tissue networks that form the supporting framework for many organs including kidney, liver, spleen, lymph nodes, Peyer’s patches in the intestine, and bone marrow—are composed of type III collagen.
Nutrivore Weekly Serving Matrix
An easy-to-use and flexible weekly checklist
to help you maximize nutrient-density.
The Weekly Serving Matrix is very helpful! I’ve been eating along these lines but this really helps me know where to focus vs. which foods serve a more secondary role. It’s super helpful and has taken a lot of worry out of my meal planning. Thanks!
Jan
Type IV collagen forms sheets instead of fibrils and is the main collagen component of the basement membrane (non-collagen components include laminin, nidogen and perlecan). The basement membrane is a thin semi-permeable noncellular layer located between epithelial or endothelial cells and the underlying connective tissue, providing structural support and a medium for signaling molecules.
Type V collagen is also fibrillar, and like type III collagen, tends to associate with type 1 collagen. Even though it’s only present in small amounts in tissues, it helps build the collagen architecture of types I and III, so it’s essential for formation of healthy collagen tissues, including in bones, corneal stroma, and the interstitial matrix of muscles, liver, lungs, and placenta.
Type IX cartilage is a FACIT cartilage (FACIT is an acronym for fibril-associated collagen with interrupted triple helices) and tends to cooperate with type II collagen in cartilage and vitreous humor, adding integrity and stability. Type IX collagen is also known to help control the diameter of collagen fibers in various tissues.
|
Our Main Collagen Types |
||
| Category | Tissues | |
| Type I | Fibrillar | bone, skin, tendons, ligaments, cornea, fibrous cartilage, connective tissue, teeth, muscle |
| Type II | Fibrillar | cartilage, vitreous body, nucleus pulposus |
| Type III | Fibrillar | skin, blood vessel, intestine, muscle, reticular fibers |
| Type IV | Network-forming collagen | basement membranes |
| Type V | Fibrillar | lung, liver, cornea, bones, placental/embryonic tissue |
| Type IX | FACIT | cartilage, vitreous humor, cornea |
Besides the above mentioned types I, II, III and V collagens, other fibrillar collagens include types XI, XXIV, and XXVII. But, collagen can form other architectures, like the above mentioned sheets formed by type IV collagen in the basement membrane. The non-fibrillar categories of collagen include network-forming collagens (including basement membrane, beaded filament-forming, anchoring fibrils, and hexagonal networks), FACIT collagens (fibril-associated collagens with interrupted triple helices) collagens, FACIT-like collagens, MACIT (membrane-associated collagens with interrupted triple helices) collagens, and multiplexin (multiple triple-helix domains and interruptions) collagens.
This diversity of collagen architectures allows collagens to perform a wide array of biomechanical functions, including its primary role of maintaining the structural integrity of tissues and organs, from forming the major functional foundation of bone and cartilage to the pericellular microenvironment that supports cellular integrity. However, collagen is a true multitasker and its roles in our bodies extend well beyond maintaining structural integrity. For example, the glomerular basement membrane (the middle layer of the selectively permeable glomerular filtration barrier in the kidneys, which stops blood proteins like albumin and globulin from leaving vasculature and entering the urinary space) is mainly composed of type IV collagen. Genetic defects in type IV collagen (such as Alport syndrome) cause glomerular kidney disease (as well as hearing loss and eye abnormalities).
Collagen also binds with specific receptors (including integrins, discoidin-domain receptors, glycoprotein VI or specialized proteoglycan receptors) to contribute to a plethora of additional biological functions, including cellular adhesion, differentiation, growth and survival! For example type I collagen binds to a peptidoglycan called decorin, thereby suppressing transforming growth factor beta (TGF-β), a multifunctional cytokine implicated in systemic inflammation and oncogenesis. Furthermore, collagen also stores and releases a variety of cellular mediators, including growth factors and cytokines, thus contributing to organ development, wound healing and tissue repair. For example, the collagenous matrix of bone binds and stores insulin-like growth factors, including insulin-like growth factor 1 (IGF-1), a major regulator of the aging process. Bones are constantly being remodeled (see The Paleo Diet for Skeletal Health), so degradation of bone by osteoclasts releases IGFs which help signal to osteoblasts to form new bone.
I think we can all agree: collagen is awesome! And here’s the rub: Normal aging, chronic inflammation, chronic stress, nutritional deficiencies, UV radiation, and smoking can all decrease collagen production as well as degrade collagen structure. This leads not only to wrinkles, but osteopenia and osteoporosis, osteoarthritis, cardiovascular disease and decreased organ function, including heart, lungs and kidneys! Yikes!
Benefits of Increasing Collagen Intake
Here’s where collagen supplements comes in. A variety of studies have shown that increasing collagen intake via supplementation can mediate the effects of aging and other factors having detrimental influence on collagen formation like inflammation and stress. In fact, collagen supplementation has been shown to improve conditions related to bone, joint, tendon, skin, muscle, and cardiovascular health!
While more studies are definitely needed to elucidate the best form of collagen and optimal dose to maximize therapeutic value for various health challenges, let’s take a look at some of the best-studied benefits collagen supplementation.
 Skin Health. Collagen is essential for skin structure and function, and is actually the decisive protein that determines skin physiology. The dry weight of young, healthy skin is at least 75% collagen, but this decreases as we age. In fact, one study measured a 68% decrease in the amount of type 1 procollagen in the skin of people over 80 years old compared to people between the ages of 18 and 29! Numerous studies have shown that collagen peptide supplementation improves skin elasticity, reducing the appearance of fine lines and wrinkles. In fact, a 2019 systematic review of eight studies showed that collagen hydrolysate supplementation at doses of 2.5 to 10 grams per day for 8 to 24 weeks showed measurable improvements in skin elasticity and moisture, as well as decreases in fine lines and wrinkles. These benefits to visible signs of skin aging are attributable to increased collagen density in the skin and a reduction in collagen fragmentation.
Skin Health. Collagen is essential for skin structure and function, and is actually the decisive protein that determines skin physiology. The dry weight of young, healthy skin is at least 75% collagen, but this decreases as we age. In fact, one study measured a 68% decrease in the amount of type 1 procollagen in the skin of people over 80 years old compared to people between the ages of 18 and 29! Numerous studies have shown that collagen peptide supplementation improves skin elasticity, reducing the appearance of fine lines and wrinkles. In fact, a 2019 systematic review of eight studies showed that collagen hydrolysate supplementation at doses of 2.5 to 10 grams per day for 8 to 24 weeks showed measurable improvements in skin elasticity and moisture, as well as decreases in fine lines and wrinkles. These benefits to visible signs of skin aging are attributable to increased collagen density in the skin and a reduction in collagen fragmentation.
 Wound Healing. Wound healing is a complex process that involves the immune system, see Nutritional Support for Injury and Wound Healing, and collagen is essential for wound healing. First, exposed collagen fibers from damaged blood vessel walls help recruit platelets to the site of injury to begin the clotting process. During the proliferative phase of wound healing, collagen is secreted by fibroblasts to form new connective tissue, providing a scaffold for contraction of the wounded area by myofibroblasts. During the remodeling phase of wound healing, collagen fibers are reorganized to return tissue to a more normal architecture. In fact, scars are largely made of collagen. One study of long-term care residents showed that pressure ulcers healed twice as fast in the group receiving a 15-gram collagen hydrolysate supplement three times daily for 8 weeks.
Wound Healing. Wound healing is a complex process that involves the immune system, see Nutritional Support for Injury and Wound Healing, and collagen is essential for wound healing. First, exposed collagen fibers from damaged blood vessel walls help recruit platelets to the site of injury to begin the clotting process. During the proliferative phase of wound healing, collagen is secreted by fibroblasts to form new connective tissue, providing a scaffold for contraction of the wounded area by myofibroblasts. During the remodeling phase of wound healing, collagen fibers are reorganized to return tissue to a more normal architecture. In fact, scars are largely made of collagen. One study of long-term care residents showed that pressure ulcers healed twice as fast in the group receiving a 15-gram collagen hydrolysate supplement three times daily for 8 weeks.
 Joint Health. The wearing down of joint cartilage in osteoarthritis causes inflexibility, pain and stiffness of predominantly weight-bearing joints such as the knees, hips and spine. Osteoarthritis is the most common form of arthritis (and is not driven by autoimmune processes like other forms of arthritis) and accounts for about 25% of primary care physician visits among the elderly. There’s accumulating evidence that collagen supplements can prevent and even reverse cartilage degradation in osteoarthritic patients. For example, one study of people with mild to moderate knee osteoarthritis showed that 10 grams of collagen hydrolysate daily over 24 weeks significantly improved a measure of cartilage quality (called the dGEMRIC score, measured by MRI) while those receiving placebo saw a continued deterioration of cartilage. Collagen supplements may improve joint health in other contexts as well. A study in athletes with activity-related joint pain showed that 10 grams daily of collagen hydrolysate for 24 weeks substantially reduced joint pain (including at rest, standing, walking, carrying objects and lifting). And in a study of general joint pain, patients receiving 1.2 grams daily of collagen hydrolysate were more likely to respond to treatments over 6 months.
Joint Health. The wearing down of joint cartilage in osteoarthritis causes inflexibility, pain and stiffness of predominantly weight-bearing joints such as the knees, hips and spine. Osteoarthritis is the most common form of arthritis (and is not driven by autoimmune processes like other forms of arthritis) and accounts for about 25% of primary care physician visits among the elderly. There’s accumulating evidence that collagen supplements can prevent and even reverse cartilage degradation in osteoarthritic patients. For example, one study of people with mild to moderate knee osteoarthritis showed that 10 grams of collagen hydrolysate daily over 24 weeks significantly improved a measure of cartilage quality (called the dGEMRIC score, measured by MRI) while those receiving placebo saw a continued deterioration of cartilage. Collagen supplements may improve joint health in other contexts as well. A study in athletes with activity-related joint pain showed that 10 grams daily of collagen hydrolysate for 24 weeks substantially reduced joint pain (including at rest, standing, walking, carrying objects and lifting). And in a study of general joint pain, patients receiving 1.2 grams daily of collagen hydrolysate were more likely to respond to treatments over 6 months.
 Muscles. Loss of muscle mass as we age, called sarcopenia, is a major cause of functional decline and loss of independence in older adults. A study of elderly sarcopenic men compared the effects on muscle mass from lifting weights three times per week with or without taking 15 grams daily of collagen peptides for 3 months. The group taking collagen gained significantly more muscle (an average gain of 4.2kg compared to 2.9kg) and lost more fat (an average loss of 5.4kg versus 3.4kg). A similar study was performed in postmenopausal women, with the collagen peptide group gaining 1.8% fat-free mass (and loss of fat mass) compared to 0.9% in the placebo group. Young healthy men can benefit from collagen supplementation too. One study in young sports students showed that those that took a 15-gram collagen peptide supplement increased muscle mass and strength more than placebo after 12-weeks of strength training. And a study of recreationally-active young men also showed similar results, with the addition of collagen peptides increasing the effectiveness of strength training over 3 months. And a study looking at vitamin C-enriched gelatin, with either 5 grams or 15 grams of gelatin, as a pre-workout supplement in healthy young men showed a dose-dependent increase in markers of collagen synthesis in their blood an hour after exercise compared to placebo, which may help to prevent musculoskeletal injuries.
Muscles. Loss of muscle mass as we age, called sarcopenia, is a major cause of functional decline and loss of independence in older adults. A study of elderly sarcopenic men compared the effects on muscle mass from lifting weights three times per week with or without taking 15 grams daily of collagen peptides for 3 months. The group taking collagen gained significantly more muscle (an average gain of 4.2kg compared to 2.9kg) and lost more fat (an average loss of 5.4kg versus 3.4kg). A similar study was performed in postmenopausal women, with the collagen peptide group gaining 1.8% fat-free mass (and loss of fat mass) compared to 0.9% in the placebo group. Young healthy men can benefit from collagen supplementation too. One study in young sports students showed that those that took a 15-gram collagen peptide supplement increased muscle mass and strength more than placebo after 12-weeks of strength training. And a study of recreationally-active young men also showed similar results, with the addition of collagen peptides increasing the effectiveness of strength training over 3 months. And a study looking at vitamin C-enriched gelatin, with either 5 grams or 15 grams of gelatin, as a pre-workout supplement in healthy young men showed a dose-dependent increase in markers of collagen synthesis in their blood an hour after exercise compared to placebo, which may help to prevent musculoskeletal injuries.
 Bones. Collagen provides the scaffold for mineralization of bones, so it’s no surprise that loss of collagen is associated with osteopenia and osteoporosis. In one study of postmenopausal women taking 5 grams of collagen peptides for a year, bone mineral density of both spine and femoral neck increased significantly compared to the placebo group. And in a study of a combined supplementation of elemental calcium, vitamin D and 5 grams of a collagen-calcium chelate for a year in osteopenic postmenopausal women, the collagen-containing supplement resulted in much less bone mineral density loss than the group receiving just calcium and vitamin D, with concurrent reduction in bloodborne markers of bone breakdown.
Bones. Collagen provides the scaffold for mineralization of bones, so it’s no surprise that loss of collagen is associated with osteopenia and osteoporosis. In one study of postmenopausal women taking 5 grams of collagen peptides for a year, bone mineral density of both spine and femoral neck increased significantly compared to the placebo group. And in a study of a combined supplementation of elemental calcium, vitamin D and 5 grams of a collagen-calcium chelate for a year in osteopenic postmenopausal women, the collagen-containing supplement resulted in much less bone mineral density loss than the group receiving just calcium and vitamin D, with concurrent reduction in bloodborne markers of bone breakdown.
The above benefits of collagen supplements are explained by two mechanism: 1) collagen supplies the specific amino acid building blocks for all of our body’s collagen proteins; and 2) bioactive peptides produced when we digest collagen (most notably prolyl‐hydroxyproline, but some larger peptides as well) upregulate the synthesis of extracellular matrix proteins in various tissues (such as by increasing growth of fibroblasts and synthesis of hyaluronic acid).
Gelatin, Collagen Hydrolysate, and Collagen Peptides, Oh My!
You may have noticed in the above section that some studies were performed using gelatin, many with collagen hydrolysate, and some with collagen peptides. So, what’s the difference?
Unprocessed, raw collagen is called native collagen. Native collagen is insoluble and therefore relatively difficult to digest (more on that below), but not many of us are gnawing on raw cartilage.
Native collagen starts to denature once its heated to a mere 40°C and is nearly fully denatured once it reaches 60°C. The heat unravels all of the collagen architecture, quaternary and tertiary structures, breaking collagen down to its constituent α-chains. Over time, some of these α-chains also break apart via hydrolysis (breaking of chemical bonds in the presence of water). Voila! This is what gelatin and bone broth protein are, denatured and partially-hydrolyzed collagen. When bone broth or gelatin cool, they gel because all those α-chain bits and pieces tend to at least partially reassemble.
We’ll get into more details on how collagen peptides are manufactured below (head’s up: they’re more processed than you may realize); but briefly, collagen peptides are made when partially-hydrolyzed collagen (i.e. gelatin) undergoes an additional step to break down the α-chain bits and pieces into even smaller polypeptides, called enzymatic hydrolysis. In this case, enzymes (like alcalase, papain, pepsin, trypsin, collagen hydrolysate, and others) are used to pre-digest the collagen. The result is called collagen hydrolysate or collagen peptides.
Here’s where terminology gets really murky: technically, gelatin is composed of hydrolyzed collagen, and technically, the term peptide refers to very short pieces of protein, chains of ten amino acids or fewer. So, while the term hydrolyzed collagen or collagen hydrolysate, which mean exactly the same thing, are often used interchangeably with collagen peptides, they can also be used to describe gelatin. Plus, with the exception of some collagen-derived pharmaceuticals, the “collagen peptides” you’re buying in the store or online are actually polypeptides.
The molecular weight of native collagen is typically 300 to 400 kiloDaltons (kDa; for reference one amino acid weighs about 110 Daltons—the math works out perfectly for three α-chains that are about 1000 amino acids long), gelatin and bone broth protein typically range from about 20kDa to 220kDa (the longer it’s heated, especially in an acidic environment, typically the shorter the resulting polypeptides), and collagen hydrolysates/peptides tend to include a wide mix of different length polypeptides in the range of 3kDa to 6kDa, and up to about 10kDa (doing the math, that’s 25ish to 100ish amino acids long).
 So, the term collagen hydrolysate, while usually used to describe enzymatically-hydrolyzed collagen, can also be used interchangeably with gelatin; and the term collagen peptides is misleading because the consumer products labeled as such are typically only enzymatically-hydrolyzed enough to make polypeptides and don’t actually contain a ton of peptides. The term collagen protein can refer to either gelatin or collagen hydrolysate. To confuse matters even more, there are also studies performed using other collagen derivatives, including low molecular weight collagen peptides (true peptides), specific bioactive collagen peptides (including dipeptides and tripeptides), and collagen tripeptides, which add to our understanding of the benefits of collagen supplementation, but represent a pharmaceutical option rather than the type of collagen we can buy in stores or online. Certainly, our digestive processes produces many of these same peptides, and studies have measured them in our bodies, but it’s not as concentrated as these pharmaceutical options. What does all this mean? a) I have to read scientific papers more carefully to know exactly what collagen derivative the researchers are using; and b) it’s not always possible to extrapolate benefits from one collagen study to the product you have in your home.
So, the term collagen hydrolysate, while usually used to describe enzymatically-hydrolyzed collagen, can also be used interchangeably with gelatin; and the term collagen peptides is misleading because the consumer products labeled as such are typically only enzymatically-hydrolyzed enough to make polypeptides and don’t actually contain a ton of peptides. The term collagen protein can refer to either gelatin or collagen hydrolysate. To confuse matters even more, there are also studies performed using other collagen derivatives, including low molecular weight collagen peptides (true peptides), specific bioactive collagen peptides (including dipeptides and tripeptides), and collagen tripeptides, which add to our understanding of the benefits of collagen supplementation, but represent a pharmaceutical option rather than the type of collagen we can buy in stores or online. Certainly, our digestive processes produces many of these same peptides, and studies have measured them in our bodies, but it’s not as concentrated as these pharmaceutical options. What does all this mean? a) I have to read scientific papers more carefully to know exactly what collagen derivative the researchers are using; and b) it’s not always possible to extrapolate benefits from one collagen study to the product you have in your home.
Incidentally, the fact that “collagen peptides” are actually polypeptides is why people who have a food allergy to the source material can still react to the hydrolyzed supplement. Antibodies typically bind to a motif around 15 to 20 amino acids long. So, if you’re allergic to beef, you may react to collagen peptides derived from cow hide.
So, to summarize:
- gelatin and bone broth protein are denatured and partially-hydrolyzed collagen
- collagen hydrolysates and collagen peptides are more fully (but not completely) hydrolyzed collagen, with enzymes used in the manufacturing process
Trying to figure out whether the collagen supplement you have is partially-hydrolyzed or is enzymatically-hydrolyzed into smaller polypeptides? Here’s the trick: if it gels (once dissolved in hot water and then chilled), it’s the former. If it dissolves easily into cold water and doesn’t gel, it’s the latter.
Food Sources of Collagen
 Wait! Let’s not forget about food! It’s absolutely possible to get supplemental levels of collagen from foods sources!
Wait! Let’s not forget about food! It’s absolutely possible to get supplemental levels of collagen from foods sources!
Collagen‐rich foods include offal, skin, joints (trotters, duck feet, chicken wings, etc.), any meat that you eat off the bone, and connective-tissue-rich cuts like cheek, jowl, and chuck roasts. See also Why Everyone Should Be Eating Organ Meat.
Bone broth (or stock) is an especially collagen-rich food to include in our diet. This flavorful elixir is made by boiling the bones (and joints and ligaments, etc.) of just about any vertebrate you can think of (typically poultry, beef, bison, lamb, pork, or fish) in water for anywhere from four hours to four days! . A 2019 study showed that long-simmered homemade bone broths—especially using the most collagen-rich tissues like beef marrow bones, chicken feet or fish heads—can deliver up to 20 grams of collagen protein in one cup of broth. Also recall that the longer collagen is heated, typically the shorter the resulting polypeptides—this is actually why a very long-simmered bone broth may not gel as much as expected, even though its very concentrated. See also Why Broth is Awesome, Broth: Hidden Dangers in a Healing Food?, and Bone Broth Risks: Skim the Fat!
Myths About Digestibility
Collagen hydrolysate and collagen peptides are often marketed as easier to digest and absorb than gelatin or bone broth protein, but while this makes sense on the surface (they’re predigested with enzymes after all), this is a myth.
Let’s briefly review protein digestion.
Digestion of protein mainly occurs in the stomach and the first section of the small intestine, the duodenum. Hydrochloric acid in the stomach denatures protein (unravels in complex structure without necessarily breaking it apart), opening up the protein’s structure for easier degradation (called proteolysis) by enzymes called proteases. Three main proteases in our body break down food proteins into polypeptides, long chains of amino acids but which are incomplete proteins: pepsin, which is secreted by the stomach, and trypsin and chymotrypsin, which are produced by the pancreas and secreted into the small intestine in reaction to hormone signals from the gut. These three proteases are each specialized in severing different types of links between specific amino acids, collectively working to degrade dietary proteins into polypeptides. Polypeptides are then broken down into peptides and individual amino acids by various peptidase enzymes, secreted by gut enterocytes (the epithelial cells which form the intestinal barrier): exopeptidases, which cleave one amino acid off of either end of a polypeptide or peptide, and dipeptidases with cleave dipeptides (a peptide made of just two amino acids) in two.
After being broken down by digestive enzymes, short peptides and amino acids are efficiently absorbed by the enterocytes of the small intestine. Approximately 30% of digested protein is absorbed as peptides, mostly tetrapeptides (four amino acids long), tripeptides (three amino acids long), and dipeptides. These peptides are endocytosed, and either transported intact, retaining biological activity, or hydrolyzed into individual amino acids inside enterocytes. The remaining 70% of digested protein is absorbed as amino acids, which are actively transported into the human body by transporters embedded in the cell membrane of the gut enterocytes, intuitively called amino acid transporters. Generally, there are multiple pathways for any given amino acid and dozens of different amino acid transporters. Rate of absorption of amino acids in the small intestine ranges from 1.3 to 10 grams per hour.
When proteins have high compatibility to our digestive processes, they tend to be close to completely broken down and absorbed before reaching the large intestine. When a protein’s structure is not particularly compatible with our digestive processes, it can reach the large intestine incompletely digested, where the proteolytic capacity of our gut bacteria can take over. If the protein is also not compatible with our gut microbiome, incompletely digested protein is expelled as part of waste. Digestibility is measured by looking at the difference between the amount of amino acids in the ingested protein versus the amount of amino acids recoverable from the, er, other end.
 It’s true that native (raw) collagen is insoluble and therefore not quite as digestible by our pancreatic enzymes as some other protein sources. One rat study from the 1980s compared the digestibility (with or without suppressing stomach acid) of native collagen and gelatin compared to meat, with whole egg as the 100% digestible standard. Native collagen was only 71% digestible when stomach acid was suppressed, but with stomach acid, its digestibility increased to 95%. Gelatin, on the other hand, was equally digestible with or without stomach acid, and its calculated true digestibility was 98.8%. For reference, the digestibility of meat in this study was 97.1%. Other studies (like this one), show similar results: unless we’re gnawing on raw chicken wings and taking huge doses of antacids, collagen and gelatin are highly digestible proteins. That means that collagen peptides do not possess a digestibility advantage over gelatin or bone broth protein.
It’s true that native (raw) collagen is insoluble and therefore not quite as digestible by our pancreatic enzymes as some other protein sources. One rat study from the 1980s compared the digestibility (with or without suppressing stomach acid) of native collagen and gelatin compared to meat, with whole egg as the 100% digestible standard. Native collagen was only 71% digestible when stomach acid was suppressed, but with stomach acid, its digestibility increased to 95%. Gelatin, on the other hand, was equally digestible with or without stomach acid, and its calculated true digestibility was 98.8%. For reference, the digestibility of meat in this study was 97.1%. Other studies (like this one), show similar results: unless we’re gnawing on raw chicken wings and taking huge doses of antacids, collagen and gelatin are highly digestible proteins. That means that collagen peptides do not possess a digestibility advantage over gelatin or bone broth protein.
There also doesn’t seem to be a big difference in how gelatin versus collagen peptides stimulate collagen synthesis once consumed. In one study, healthy young men were given placebo, a supplement containing 15 grams of gelatin, a supplement containing 15 grams of hydrolyzed collagen, or a supplement containing 7.5 grams each of gelatin and hydrolyzed collagen. An hour after consuming the collagen, participants jumped rope for 6 minutes to stimulate endogenous collagen synthesis. Four hours later, blood was drawn and markers of collagen synthesis were measured. The study revealed no significant difference between the collagen-derived amino acids circulating in the blood between the three different collagen supplements, although all were substantially higher than placebo (including approximately doubling the serum amount of glycine, proline, hydroxyproline and hydroxylysine). And, circulating procollagen was 20% higher after gelatin or hydrolyzed collagen compared to placebo. So, from a supporting-collagen-synthesis-in-our-bodies perspective, gelatin and hydrolyzed collagen perform equally well.
And, there’s another really important consequence of this information: the bioactive peptides found in pharmaceutical collagen peptides are a natural product of our digestion, albeit not as concentrated. There may still be additional benefit to pharmaceutical-grade specific collagen peptides as a medication, but there’s no digestibility or bioavailability advantage to collagen peptides (despite label claims) compared to gelatin or bone broth protein.
How Collagen Supplements Are Made
Okay, let’s dig into the good, the bad, and the ugly on how various collagen supplements are made.
Gelatin – The O.G. Collagen Supplement

Gelatin dates back to Ancient Rome, when it was used to make glue. The oldest recipes for aspics and jellies—gelatin-based savory dishes—date to the early 1400s. At that time, gelatin was made much the way we make a rich homemade bone broth today. Calf or pig feet, or pig ears, were boiled in water in a large kettle over a fire for many hours, after which the liquid was strained and the bones discarded. As it cooled, the gelatin would set with the fat rising to the top and solidifying. The fat was then be skimmed off and the gelatin was used in a dish. It was a process that typically took at least two days. Packaged gelatin was first available in the 1840s, which Charles Knox began packaging dried sheets of gelatin and hired traveling salesman to sell it door-to-door. Jell-O first hit the stage in 1897.
Commercial gelatin is now made via a relatively simple but industrial process with several steps. Byproducts of the meat industry—including animal hides, bones, and other tissues, are transported the usually short distance from abattoir to food processing plant. After inspection, the tissues are loaded into chopping machines that cut them down to pieces a few inches in diameter. Next, the pieces are washed under high-pressure to remove any debris. They are then soaked in hot water followed by roasting at 100°C for 30 minutes to degrease and begin denaturing the collagen. Sometimes solvents are used in this step, either hexane or chloroform-methane, but not always, and these are not allowed if the final product will be USDA Organic. Typically, the pieces are then soaked in either an acid or alkaline solution for five days, which demineralizes, disinfects, and facilitates collagen release. (If acid is used, either acetic acid or hydrochloric acid is typical; if alkaline is used, potassium carbonate, sodium carbonate or sodium hydroxide are typical.) Next, the mixture is loaded into large extractors and boiled in distilled water while a tube running off the extractor draws off the liquid that now contains gelatin. After a flash-heating to sterilize, the liquid is piped through filters (usually activated carbon, and a frame filter) to deodorize, decolorize and purify it. Finally, the liquid is piped into evaporators to remove the water, and the gelatin is pressed into sheets before being ground into a fine powder.
Gelatin is nearly tasteless, dissolves in hot liquid, and gels once that liquid is cooled again. Because it’s 99% protein, one tablespoon of gelatin powder has 6 grams of protein, albeit an incomplete protein. Gelatin and all collagen derivatives lack one indispensable amino acid (tryptophan) and therefore have a Protein Digestibility-Corrected Amino Acid Score (PDCAAS) and Digestible Indispensable Amino Acid Score (DIAAS) of zero! That’s because these measures of protein digestibility correct for the presence of essential amino acids. The exact amino acid breakdown of gelatin (and indeed collagen peptides) is dependent on the source material, but it’s typical for gelatin to be predominantly glycine (26-34%), proline (10-18%), and hydroxyproline (7-15%) with notable amounts of alanine (8-11%), arginine (8-9%), aspartic acid (6-7%), and glutamic acid (10-12%).
Yes, as you can probably deduce, there’s a range from quite natural processes (without solvents and using acetic acid, see The Health Benefits of Apple Cider Vinegar) for the production of gelatin to much less so. Unfortunately, most companies view their process as proprietary and are quite opaque about which (if any) chemicals are used in production. And, the same is true for collagen peptides…
Collagen Peptides
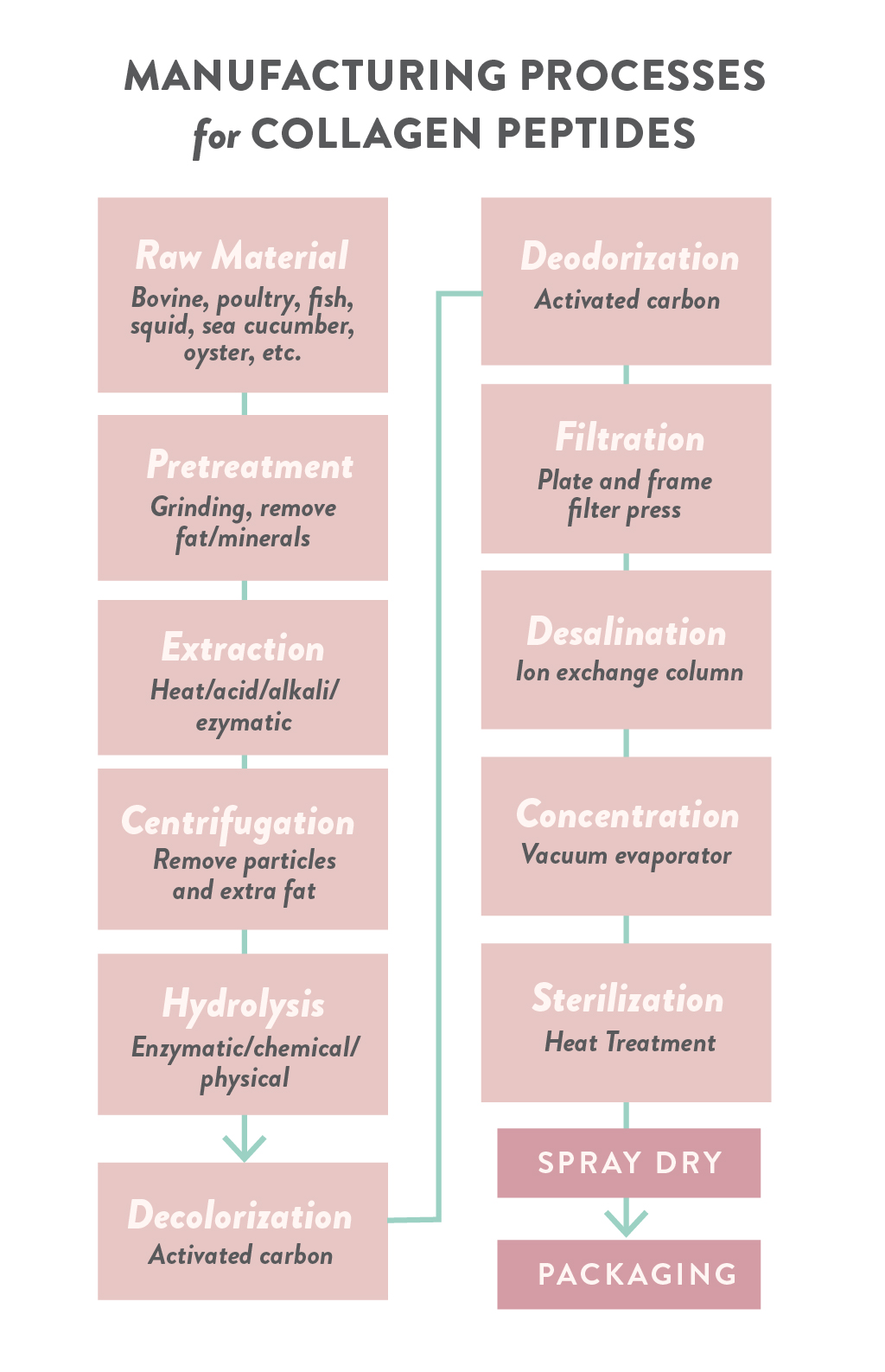
Collagen peptides (aka hydrolyzed collagen, collagen hydrolysate, gelatin hydrolysate, and collagen protein) are made similarly to gelatin, but with the addition of an enzymatic hydrolysis step after extraction. The extracted gelatin is mixed with enzymes (most common are papain, alcalase, α-chymotrypsin, pepsin, trypsin, collagenase, and bromelain). Sometimes enzymes are added concurrently with acid treatment, the most common combination being acetic acid and pepsin. The length of this process depends on the proprietary enzyme mix being used. After enzymatic hydrolysis, the same filtering, concentrating, drying and grinding as with gelatin are performed. This 2019 review paper has a very good summary of the various manufacturing processes that are used to make collagen peptides.
Like gelatin, hydrolyzed collagen is near tasteless, but unlike gelatin, it does not form a gel when dissolved in liquid and cooled, even in high concentrations. As a result, collagen peptides can be easily dissolved into either hot or cold water, making them a more versatile supplement. Again, the amino acid profile is reflective of the source material (most commonly bovine or porcine hides), so there’s little difference from gelatin.
Recall that collagen peptides (which are really polypeptides) do not offer a digestibility or bioavailability compared to gelatin or bone broth protein. That means the only advantage this supplement has to offer is the convenience of stirring it into cold liquids.
What About Marine Collagen?
Marine collagen is typically extracted from fish scales, following a comparable procedure to collagen peptides extracted from bovine, porcine hides or animal byproducts. However, the amino acid profile is slightly different, being a bit higher in glycine and lower in both proline and hydroxyproline. This may be helpful if your primary reason for taking a collagen supplement is to increase glycine intake.
Glycine is required for synthesis of DNA, RNA and many proteins in the body. As such, it plays extensive roles in digestive health, proper functioning of the nervous system and in wound healing. Glycine aids digestion by helping to regulate the synthesis and of bile salts and secretion of gastric acid. It is involved in detoxification and is required for production of glutathione, an important antioxidant. Glycine helps regulate blood sugar levels by controlling gluconeogenesis (the manufacture of glucose from proteins in the liver). Glycine also enhances muscle repair/growth by increasing levels of creatine and regulating Human Growth Hormone secretion from the pituitary gland. This wonderful amino acid is also critical for healthy functioning of the central nervous system. In the brain, it inhibits excitatory neurotransmitters, thus producing a calming effect. Glycine is also converted into the neurotransmitter serine, which promotes mental alertness, improves memory, boosts mood, and reduces stress. Glycine is conditionally indispensable, meaning that while we can make our own, there are many circumstances where we can’t make sufficient glycine to meet our body’s needs.
How much more glycine does marine collagen offer? Not a whole lot, and it depends on the exact source. One major brand’s marine collagen is 24.1% glycine whereas their bovine hide collagen is 20.7%. Another major brand’s marine collagen is 22.7% glycine whereas their bovine hide collagen is 23.3%. If increasing glycine is the only reason you’re supplementing with collagen and these small differences are important to you, then a lot of label reading and a bit of math is in your future. Another reason to choose marine collagen is an allergy to beef or pork (and not to fish), because as we’ve already established the polypeptides in collagen products are still long enough to trigger allergic responses.
Is Multi-Collagen Worth It?
Multi-collagen supplements typically include several hydrolyzed collagens from various sources, including bovine or porcine hide, egg membrane collagen, hydrolyzed fish collagen (aka marine collagen), and bone broth protein (which may or may not be hydrolyzed).
Importantly, there is not a one-to-one correspondence between the type of collagen consumed and the type of collagen your body makes. In fact, the only appreciable difference is a slight shift in amino acid profile reflecting the amino acid composition of the source materials. Because collagen is so readily digested, your body is still mostly absorbing the constituent amino acids. And so far, scientific studies have not identified any special bioactive peptides in more expensive collagen hydrolysate ingredients found in multi-collagen supplements to justify the increased expense.
Some whole food sources of collagen can contain added benefits though; for example, sources of type 2 collagen (when minimally processed, such as tracheal cartilage) can also be rich in glucosamine chondroitin, a nutrient well-established to support joint health. To be clear, the benefit of these supplements is the additional molecules in those tissues and not the different type of collagen itself.
Bone Broth Protein
Bone broth protein is dehydrated bone broth, a traditional food whose healing properties can be attributed to its collagen content. See also Why Broth is Awesome, Broth: Hidden Dangers in a Healing Food?, and Bone Broth Risks: Skim the Fat!
If the label says hydrolyzed bone broth protein or hydrolyzed bone broth collagen, and especially if the label also says you can stir the powder into cold beverages, then this is a very similarly-processed product to collagen peptides (enzymatic hydrolysis and all), with the difference being that the source material is boiled bones rather than other sources of collagen, like bovine or porcine hide.
 However, if the label says simply bone broth protein or bone broth collagen (without the word “hydrolyzed”), then you have the least processed option for a collagen supplement! Congratulations!
However, if the label says simply bone broth protein or bone broth collagen (without the word “hydrolyzed”), then you have the least processed option for a collagen supplement! Congratulations!
I personally use Paleovalley 100% Grass-Fed Bone Broth Protein, which is quite simply the cleanest source of collagen that I know of! It’s made by slow-simmering 100% grass-fed beef bones in filtered water, just like I would at home, then it’s gently powdered with no additives. It’s never treated with chemicals or high-heat, and it’s third-party tested for contaminants. You just can’t get a higher-quality, or more convenient, source of collagen.
I add Paleovalley Bone Broth Protein to my morning coffee (it’s better stirred in rather than blended since it tends to foam a lot if you blend it, side effect of the longer collagen polypeptides, although it doesn’t gel firmly like gelatin), and I also add it to smoothies (at the end with just a quick whirr to incorporate), soups, stews, stir fries and even baking. It has very little flavor (I can’t taste it at all in my coffee), but you can turn it into a warm cup of broth with it by mixing into hot water and adding salt to taste.
Save 15% with code THEPALEOMOM15
There’s also a huge advantage of consuming bone broth protein rather than homemade bone broth: concentration and consistency. In fact, a 2019 study showed that the amino acid content of common homemade preparations of long-simmered bone broth varied wildly, with the standard cup (250mL) of bone broth delivering as little as 10% of the collagen protein of the usual 20-gram supplement. (Incidentally, the most concentrated broths in the study were those made with beef marrow bones simmered for 72 hours, with the addition of vinegar having a negligible effect on amino acid extraction. These broths delivered close to parity amounts of collagen protein in a one-cup serving. Good news for our favorite traditional healing food!) Bone broth protein on the other hand, is standardized and concentrated, so you know you’re getting the right dose every time!
I still use homemade bone broth for soups, stews and other recipes that call for broth, but choose Paleovalley Bone Broth Protein for my daily collagen supplement.
Reading Labels and Icky Ingredients
To summarize, bone broth protein (not hydrolyzed) is the clear winner when it comes to collagen supplementation. But, there are some other important things to look for on a label.
In one recent ConsumerLab.com test of 14 popular collagen supplements, one contained high levels of the heavy metal cadmium. This highlights the importance of looking for a brand that does third-party testing (like Paleovalley does!) to verify that there are no heavy metals or other contaminants in their finished product.
Also, there are a variety of questionable if not outright icky ingredients added to collagen products as flavorings, preservatives, anticaking agents, emulsifiers, etc. Any of the ingredients below are a reason for me to put the product back on the shelf and move on:
- Sweeteners – stevia, monk fruit, erythritol, xylitol, see The Trouble with Stevia, What’s the Next Superfood Sweetener? and Is It Paleo? Splenda, Erythritol, Stevia and other low-calorie sweeteners
- Emulsifiers – xanthum gum, guar gum, cellulose gum, hypromellose (hydroxypropyl methylcellulose), see Is It Paleo? Guar Gum, Xanthan Gum and Lecithin, Oh My!
- Anticaking agents – maltodextrin, tricalcium phosphate, silica or silicon dioxide
- Preservatives – sodium benzoate or benzoic acid
- Manufacturing aids – magnesium stearate or stearic acid
- Supplements – hyaluronic acid (often wheat derived and may contain trace amount of gluten, check source and transparency from company), adaptogens, etc.
I always choose an unflavored collagen supplement with absolutely no added ingredients. Yet another check in the pro column for my favorite, Paleovalley 100% Grass-Fed Bone Broth Protein.
How Much Collagen to Take
 Phew! That was a long road to collagen is awesome, bone broth and bone broth protein are the best sources, and we can pretty much all benefit from incorporating more of it into our daily routines. So, let’s wrap up on the obvious last question: How much?
Phew! That was a long road to collagen is awesome, bone broth and bone broth protein are the best sources, and we can pretty much all benefit from incorporating more of it into our daily routines. So, let’s wrap up on the obvious last question: How much?
Most studies showing benefit of collagen supplementation, whether gelatin or collagen hydrolysate, used doses between 10 and 20 grams daily. However, you can consume quite a lot more than that without jeopardizing the amino acid balance of your diet as a whole. Recall that collagen is an incomplete protein completely lacking in the essential amino acid tryptophan, which is why it has a PDCAAS of zero even though its highly (98.8%) digestible. Researchers have used iterative PDCAAS calculations to show that collagen peptides can make up to 36% of our dietary protein while ensuring indispensable amino acid requirements are met. That means that if you’re aiming for 150 grams of protein daily, you can safely get a little over 50 grams of that from collagen! For reference, that’s three heaping scoops of Paleovalley 100% Grass-Fed Bone Broth Protein!
Citations
Alcock RD, Shaw GC, Burke LM. Bone Broth Unlikely to Provide Reliable Concentrations of Collagen Precursors Compared With Supplemental Sources of Collagen Used in Collagen Research. Int J Sport Nutr Exerc Metab. 2019 May 1;29(3):265-272. doi: 10.1123/ijsnem.2018-0139.
Barati M, Jabbari M, Navekar R, Farahmand F, Zeinalian R, Salehi-Sahlabadi A, Abbaszadeh N, Mokari-Yamchi A, Davoodi SH. Collagen supplementation for skin health: A mechanistic systematic review. J Cosmet Dermatol. 2020 May 21. doi: 10.1111/jocd.13435.
Bello AE, Oesser S. Collagen hydrolysate for the treatment of osteoarthritis and other joint disorders: a review of the literature. Curr Med Res Opin. 2006 Nov;22(11):2221-32. doi: 10.1185/030079906X148373.
Bruyère O, Zegels B, Leonori L, Rabenda V, Janssen A, Bourges C, Reginster JY. Effect of collagen hydrolysate in articular pain: a 6-month randomized, double-blind, placebo controlled study. Complement Ther Med. 2012 Jun;20(3):124-30. doi: 10.1016/j.ctim.2011.12.007.
Choi FD, Sung CT, Juhasz ML, Mesinkovsk NA. Oral Collagen Supplementation: A Systematic Review of Dermatological Applications. J Drugs Dermatol. 2019 Jan 1;18(1):9-16.
Clark K.L., Sebastianelli W., Flechsenhar K.R., Aukermann D.F., Meza F., Millard R.L., Deitch J.R., Sherbondy P.S., Albert A. 24-Week study on the use of collagen hydrolysate as a dietary supplement in athletes with activity-related joint pain. Curr. Med. Res. Opin. 2008;24:1485–1496. doi: 10.1185/030079908X291967.
Coffey JW, Fiedler-Nagy C, Georgiadis AG, Salvador RA. Digestion of native collagen, denatured collagen, and collagen fragments by extracts of rat liver lysosomes. J Biol Chem. 1976 Sep 10;251(17):5280-2.
de Almagro MC. The Use of Collagen Hydrolysates and Native Collagen in Osteoarthritis. 2020; 7(6). AJBSR.MS.ID.001217. doi: 10.34297/AJBSR.2020.07.001217.
Elam ML, Johnson SA, Hooshmand S, Feresin RG, Payton ME, Gu J, Arjmandi BH. A calcium-collagen chelate dietary supplement attenuates bone loss in postmenopausal women with osteopenia: a randomized controlled trial. J Med Food. 2015 Mar;18(3):324-31. doi: 10.1089/jmf.2014.0100.
Fu Y, Therkildsen M, Aluko RE, Lametsch R. Exploration of collagen recovered from animal by-products as a precursor of bioactive peptides: Successes and challenges. Crit Rev Food Sci Nutr. 2019;59(13):2011-2027. doi: 10.1080/10408398.2018.1436038.
Gelse K, Pöschl E, Aigner T. Collagens–structure, function, and biosynthesis. Adv Drug Deliv Rev. 2003 Nov 28;55(12):1531-46. doi: 10.1016/j.addr.2003.08.002.
Harkness ML, Harkness RD, Venn MF. Digestion of native collagen in the gut. Gut. 1978 Mar;19(3):240-3. doi: 10.1136/gut.19.3.240.
Hong H, Fan H, Chalamaiah M, Wu J. Preparation of low-molecular-weight, collagen hydrolysates (peptides): Current progress, challenges, and future perspectives. Food Chem. 2019 Dec 15;301:125222. doi: 10.1016/j.foodchem.2019.125222.
Horn MA, Trafford AW. Aging and the cardiac collagen matrix: Novel mediators of fibrotic remodelling. J Mol Cell Cardiol. 2016 Apr; 93: 175–185. doi: 10.1016/j.yjmcc.2015.11.005
https://www.grandviewresearch.com/press-release/global-collagen-market
Iwai K, Hasegawa T, Taguchi Y, Morimatsu F, Sato K, Nakamura Y, Higashi A, Kido Y, Nakabo Y, Ohtsuki K. Identification of food-derived collagen peptides in human blood after oral ingestion of gelatin hydrolysates. J Agric Food Chem. 2005 Aug 10;53(16):6531-6. doi: 10.1021/jf050206p.
Jendricke P, Centner C, Zdzieblik D, Gollhofer A, König D. Specific Collagen Peptides in Combination with Resistance Training Improve Body Composition and Regional Muscle Strength in Premenopausal Women: A Randomized Controlled Trial. Nutrients. 2019 Apr 20;11(4):892. doi: 10.3390/nu11040892.
Kim DU, Chung HC, Choi J, Sakai Y, Lee BY. Oral Intake of Low-Molecular-Weight Collagen Peptide Improves Hydration, Elasticity, and Wrinkling in Human Skin: A Randomized, Double-Blind, Placebo-Controlled Study. Nutrients. 2018 Jun 26;10(7):826. doi: 10.3390/nu10070826.
Kirmse M, Oertzen-Hagemann V, de Marées M, Bloch W, Platen P. Prolonged Collagen Peptide Supplementation and Resistance Exercise Training Affects Body Composition in Recreationally Active Men. Nutrients. 2019 May 23;11(5):1154. doi: 10.3390/nu11051154.
Kleinnijenhuis AJ, van Holthoon FL, Maathuis AJH, Vanhoecke B, Prawitt J, Wauquier F, Wittrant Y. Non-targeted and targeted analysis of collagen hydrolysates during the course of digestion and absorption. Anal Bioanal Chem. 2020 Feb;412(4):973-982. doi: 10.1007/s00216-019-02323-x.
König D, Oesser S, Scharla S, Zdzieblik D, Gollhofer A. Specific Collagen Peptides Improve Bone Mineral Density and Bone Markers in Postmenopausal Women-A Randomized Controlled Study. Nutrients. 2018 Jan 16;10(1):97. doi: 10.3390/nu10010097.
Lee SK, Posthauer ME, Dorner B, Redovian V, Maloney MJ. Pressure ulcer healing with a concentrated, fortified, collagen protein hydrolysate supplement: a randomized controlled trial. Adv Skin Wound Care. 2006 Mar;19(2):92-6. doi: 10.1097/00129334-200603000-00011.
León-López A, Morales-Peñaloza A, Martínez-Juárez VM, Vargas-Torres A, Zeugolis DI, Aguirre-Álvarez G. Hydrolyzed Collagen-Sources and Applications. Molecules. 2019 Nov 7;24(22):4031. doi: 10.3390/molecules24224031.
Lis DM, Baar K. Effects of Different Vitamin C-Enriched Collagen Derivatives on Collagen Synthesis. Int J Sport Nutr Exerc Metab. 2019 Sep 1;29(5):526-531. doi: 10.1123/ijsnem.2018-0385.
Liu D, Nikoo M, Boran G, Zhou P, Regenstein JM. Collagen and gelatin. Annu Rev Food Sci Technol. 2015;6:527-57. doi: 10.1146/annurev-food-031414-111800.
McAlindon T.E., Nuite M., Krishnan N., Ruthazer R., Price L.L., Burstein D., Griffith J., Flechsenhar K. Change in knee osteoarthritis cartilage detected by delayed gadolinium enhanced magnetic resonance imaging following treatment with collagen hydrolysate: A pilot randomized controlled trial. Osteoarthr. Cartil. 2011;19:399–405. doi: 10.1016/j.joca.2011.01.001.
Meschiari CA, Ero OK, Pan H, Finkel T, Lindsey ML. The impact of aging on cardiac extracellular matrix. Geroscience. 2017 Feb;39(1):7-18. doi: 10.1007/s11357-017-9959-9.
Mohammad AW, Suhimi NM, Aziz AGKA, Jahim JM. Process for Production of Hydrolysed Collagen from Agriculture Resources: Potential for Further Development. Journal of Applied Sciences, 2014;14: 1319-1323. doi: 10.3923/jas.2014.1319.1323
Oertzen-Hagemann V, Kirmse M, Eggers B, Pfeiffer K, Marcus K, de Marées M, Platen P. Effects of 12 Weeks of Hypertrophy Resistance Exercise Training Combined with Collagen Peptide Supplementation on the Skeletal Muscle Proteome in Recreationally Active Men. Nutrients. 2019 May 14;11(5):1072. doi: 10.3390/nu11051072.
Ohara H, Matsumoto H, Ito K, Iwai K, Sato K. J Agric Food Chem. 2007 Feb 21;55(4):1532-5. doi: 10.1021/jf062834s. Comparison of quantity and structures of hydroxyproline-containing peptides in human blood after oral ingestion of gelatin hydrolysates from different sources. Epub 2007 Jan 25.
Osawa Y, Mizushige T, Jinno S, Sugihara F, Inoue N, Tanaka H, Kabuyama Y. Absorption and metabolism of orally administered collagen hydrolysates evaluated by the vascularly perfused rat intestine and liver in situ. Biomed Res. 2018;39(1):1-11. doi: 10.2220/biomedres.39.1.
Panwar P, Lamour G, Mackenzie NC, Yang H, Ko F, Li H, Brömme D. Changes in Structural-Mechanical Properties and Degradability of Collagen during Aging-associated Modifications. J Biol Chem. 2015 Sep 18;290(38):23291-306. doi: 10.1074/jbc.M115.644310.
Paul C, Leser S, Oesser S. Significant Amounts of Functional Collagen Peptides Can Be Incorporated in the Diet While Maintaining Indispensable Amino Acid Balance. Nutrients. 2019 May 15;11(5):1079. doi: 10.3390/nu11051079.
Proksch E., Segger D., Degwert J., Schunck M., Zague V., Oesser S. Oral supplementation of specific collagen peptides has beneficial effects on human skin physiology: A double-blind, placebo-controlled study. Skin. Pharmacol. Physiol. 2014;27:47–55. doi: 10.1159/000351376.
Reuterswärd, AL, Fabiansson, S. In vivo digestibility of insoluble collagen from bovine tendon as influenced by the inhibition of gastric acid secretion. Journal of Food Science, 2010; 50(6), 1523–1525. Doi: 10.1111/j.1365-2621.1985.tb10524.x
Ricard-Blum S, Ruggiero F. The collagen superfamily: from the extracellular matrix to the cell membrane. Pathol Biol (Paris). 2005 Sep;53(7):430-42. doi: 10.1016/j.patbio.2004.12.024.
Ricard-Blum S. The collagen family. Cold Spring Harb Perspect Biol. 2011 Jan 1;3(1):a004978. doi: 10.1101/cshperspect.a004978.
Sato K. The presence of food-derived collagen peptides in human body-structure and biological activity. Food Funct. 2017 Dec 13;8(12):4325-4330. doi: 10.1039/c7fo01275f.
Shaw G, Lee-Barthel A, Ross ML, Wang B, Baar K. Vitamin C-enriched gelatin supplementation before intermittent activity augments collagen synthesis. Am J Clin Nutr. 2017 Jan;105(1):136-143. doi: 10.3945/ajcn.116.138594.
Song H, Zhang S, Zhang L, Li B. Effect of Orally Administered Collagen Peptides from Bovine Bone on Skin Aging in Chronologically Aged Mice. Nutrients. 2017 Nov; 9(11): 1209. doi: 10.3390/nu9111209
Sugihara F, Inoue N, Venkateswarathirukumara S. Ingestion of bioactive collagen hydrolysates enhanced pressure ulcer healing in a randomized double-blind placebo-controlled clinical study. Sci Rep. 2018 Jul 30;8(1):11403. doi: 10.1038/s41598-018-29831-7.
Taga Y, Iwasaki Y, Shigemura Y, Mizuno K. Improved in Vivo Tracking of Orally Administered Collagen Hydrolysate Using Stable Isotope Labeling and LC-MS Techniques. J Agric Food Chem. 2019 Apr 24;67(16):4671-4678. doi: 10.1021/acs.jafc.9b00571.
Varani J, Dame MK, Rittie L, Fligiel SE, Kang S, Fisher GJ, Voorhees JJ. Decreased collagen production in chronologically aged skin: roles of age-dependent alteration in fibroblast function and defective mechanical stimulation. Am J Pathol. 2006 Jun;168(6):1861-8. doi: 10.2353/ajpath.2006.051302.
Zdzieblik D, Oesser S, Baumstark MW, Gollhofer A, König D. Collagen peptide supplementation in combination with resistance training improves body composition and increases muscle strength in elderly sarcopenic men: a randomised controlled trial. Br J Nutr. 2015 Oct 28;114(8):1237-45. doi: 10.1017/S0007114515002810.
Zhu S, Huang M, Feng G, Miao Y, Wu H, Zeng M, Lo YM. Gelatin versus its two major degradation products, prolyl-hydroxyproline and glycine, as supportive therapy in experimental colitis in mice. Food Sci Nutr. 2018 Apr 16;6(4):1023-1031. doi: 10.1002/fsn3.639.














