One of the core principles of a Paleo diet is avoiding foods that contain compounds called toxic lectins. The term toxic lectin originates from the fact that lectins are a class of carbohydrate-binding proteins found in all foods (and indeed all forms of life, including humans!), but that only a subset of these are an issue for human health via food consumption (hence lectins versus toxic lectins). The lectins that are of concern have a few important properties: they are relatively stable through food preparation; they are difficult for humans to digest; they are known to interact strongly with the brush border of the intestine (which may impact cell viability and/or barrier function in addition to allowing protected transport of the toxic lectin into the body); and they are biologically active once they enter the body. There are two main classes of these toxic lectins: prolamins and agglutinins. Agglutinins will be discussed in another post.
One of the core principles of a Paleo diet is avoiding foods that contain compounds called toxic lectins. The term toxic lectin originates from the fact that lectins are a class of carbohydrate-binding proteins found in all foods (and indeed all forms of life, including humans!), but that only a subset of these are an issue for human health via food consumption (hence lectins versus toxic lectins). The lectins that are of concern have a few important properties: they are relatively stable through food preparation; they are difficult for humans to digest; they are known to interact strongly with the brush border of the intestine (which may impact cell viability and/or barrier function in addition to allowing protected transport of the toxic lectin into the body); and they are biologically active once they enter the body. There are two main classes of these toxic lectins: prolamins and agglutinins. Agglutinins will be discussed in another post.
What are Prolamins?
Prolamins are a toxic lectin that are abundant in grains, legumes, and pseudo-grains (more specifically, in the seed of the plant, which includes wheat, oats, barley, quinoa, rice, peanuts, and soy).
Gluten is the best-known example of a prolamin, the most thoroughly studied, and is also the most dangerous. Most recently, the term glutenoid has been coined to emphasize the connection between other members of this glycoprotein family and the effects of gluten. Much of the current understanding of the effect that prolamins have on the gut barrier, but also on the overall immune system, comes from studies of gluten (and more specifically, the protein component of gluten, which is called gliadin), often conducted in the context of celiac disease. Although glutenoid has yet to catch on, when it does it may be easier for people to understand that there are similar proteins in nongluten-containing grains as well as pseudo-grains and legumes that are similarly problematic for health.
Prolamins function as storage proteins in plants and are the major source of important proteins for seed germination. In fact, prolamins account for approximately half the total protein in all grains. There are many different prolamins, all characterized by their high content of the amino acid proline. Examples of prolamins are gliadin in wheat (gliadin is the protein fraction of gluten), hordein in barley, secalin in rye, zein in corn, kafirin in sorghum, orzenin in rice, and avenin in oats.
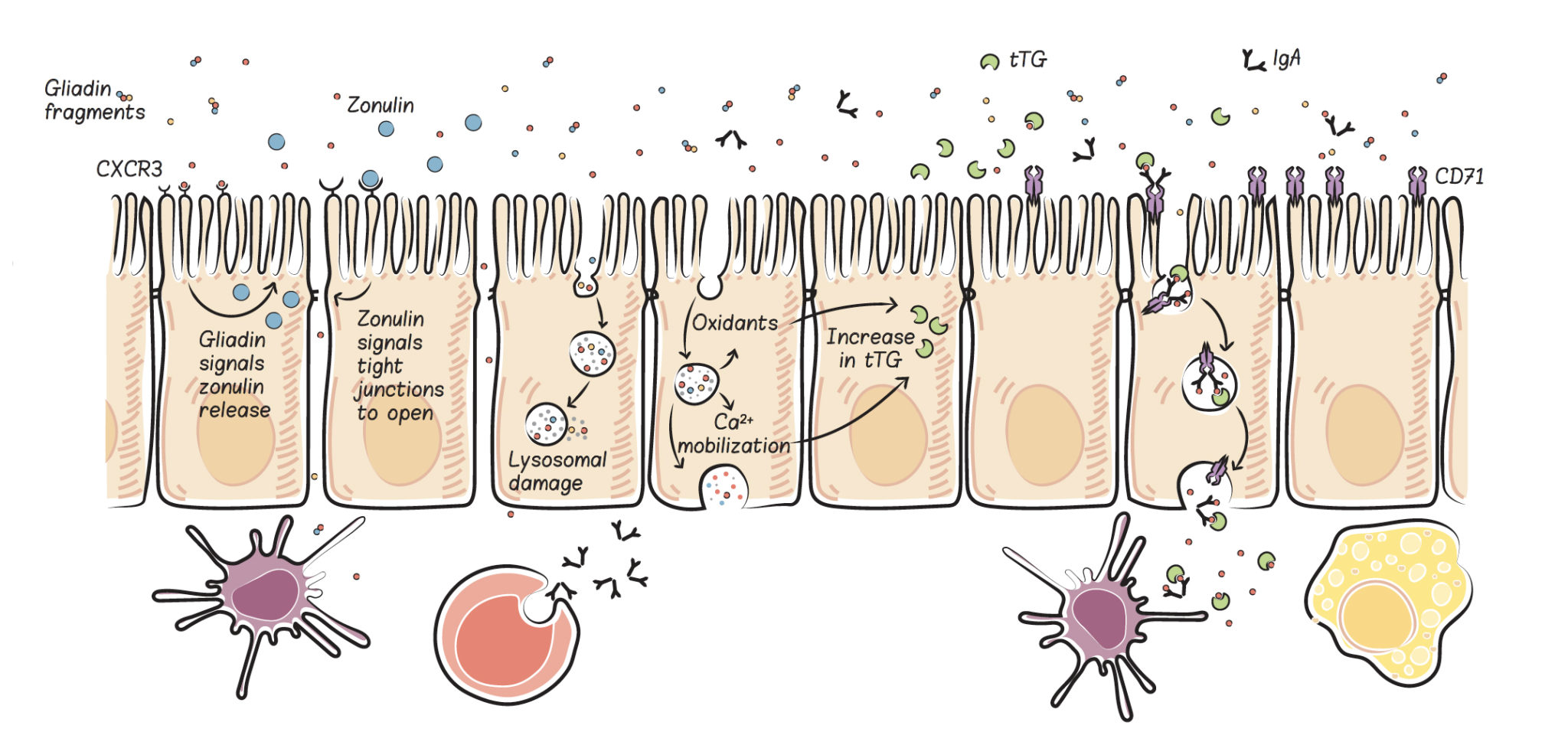
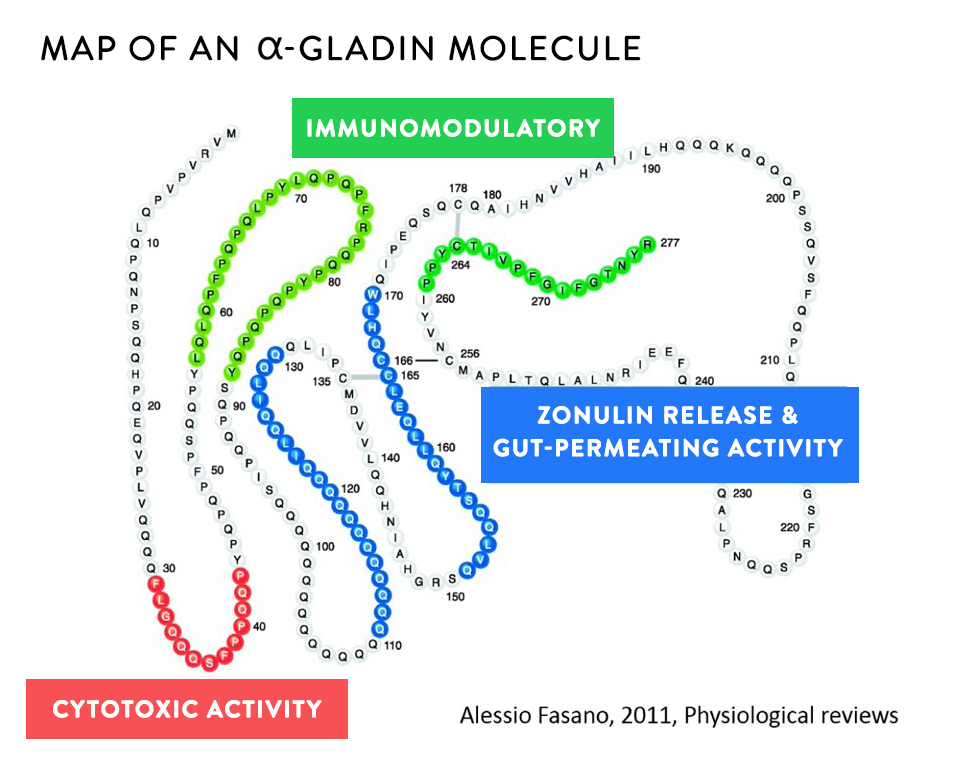 Usually food proteins are degraded into very small peptides or individual amino acids by digestive enzymes (called peptidases or proteases) before they are transported across the gut epithelium. However, prolamins are not completely broken down in the normal digestive process, both because the structure of these proteins is not compatible with our digestive enzymes (which are not good at breaking apart proline-rich proteins into individual amino acids) but also because the seeds that contain prolamins also contain protease inhibitors (compounds that stop our enzymes from breaking down proteins, a part of the seed’s natural defense mechanisms, discussed more in Wheat and Innate Immunity and How Do Grains, Legumes and Dairy Cause a Leaky Gut? Part 2: Saponins and Protease Inhibitors). Therefore, many prolamin fragments travel though the digestive tract but are also able to cross the gut barrier largely intact, and in doing so, damage the barrier and cause a leaky gut.
Usually food proteins are degraded into very small peptides or individual amino acids by digestive enzymes (called peptidases or proteases) before they are transported across the gut epithelium. However, prolamins are not completely broken down in the normal digestive process, both because the structure of these proteins is not compatible with our digestive enzymes (which are not good at breaking apart proline-rich proteins into individual amino acids) but also because the seeds that contain prolamins also contain protease inhibitors (compounds that stop our enzymes from breaking down proteins, a part of the seed’s natural defense mechanisms, discussed more in Wheat and Innate Immunity and How Do Grains, Legumes and Dairy Cause a Leaky Gut? Part 2: Saponins and Protease Inhibitors). Therefore, many prolamin fragments travel though the digestive tract but are also able to cross the gut barrier largely intact, and in doing so, damage the barrier and cause a leaky gut.
How Do Prolamins (like Gluten) Cross the Gut Barrier?
Most of the current understanding of how prolamins cross and damage the gut barrier comes from studies of various gliadin fragments (which are commonly formed when gluten is partly digested by our proteases). Several fragments of gliadin have been well-characterized in terms of their effect on the gut barrier and their ability to activate both the innate and the adaptive immune systems. Gliadin fragments can cross the gut barrier through one of two pathways:
- paracellular—in between the cells that line the gut
- transcellular—through the cells that line the gut (which encompasses multiple routes)
In some people, one route or the other will dominate (probably dependent on genetic factors). For others, both pathways are important contributors to a leaky gut. And while it doesn’t really matter which way prolamins are causing your gut to be leaky, understanding these pathways is important for grasping the necessity of avoiding grains, pseudo-grains, and legumes. See also Grains: What Are They?, Are Pseudograins Pseudobad?, 3 Myths About Legumes — Busted!, The Green Bean Controversy and Pea-Gate, What Is A Leaky Gut? (And How Can It Cause So Many Health Issues?), What Should You Eat To Heal a Leaky Gut?, and 8 Nutrients for Leaky Gut.
Quick Review of Gut Cell Biology
The gut is a barrier between the inside of your body and the outside world. Yes, as unintuitive as it may be, the stuff inside your digestive tract is actually outside your body. But, the gut is a very unique barrier. Its job is to let important nutrients inside the body while keeping everything else out. This makes it a highly selective semi-permeable barrier. Nutrients enter the body through a variety of tightly controlled mechanisms.
What forms this highly selective semi-permeable barrier is a single layer of highly specialized cells called gut epithelial cells, or enterocytes.
 Epithelial cells are amazing (I happen to be very fond of them in large part because the last two years of medical research I did before having my first daughter was studying these guys!). They are one of a few types of cells within the human body that have a top and a bottom. The structure of the cell membrane is different at the top of the cell compared to the sides and bottom. At the top (also called the apical side of the cell), the outer cell membrane is shaped into thousands of fingerlike projections called microvili which act to increase the surface area of the top of the cell. The membrane that forms the sides and bottom of the cell (also called the basolateral membrane) is smooth. These two membranes have different jobs to do. The apical membrane faces inside the gut, and has the very important job of engulfing nutrients to transport through the cell, across the basolateral membrane, and then into the body.
Epithelial cells are amazing (I happen to be very fond of them in large part because the last two years of medical research I did before having my first daughter was studying these guys!). They are one of a few types of cells within the human body that have a top and a bottom. The structure of the cell membrane is different at the top of the cell compared to the sides and bottom. At the top (also called the apical side of the cell), the outer cell membrane is shaped into thousands of fingerlike projections called microvili which act to increase the surface area of the top of the cell. The membrane that forms the sides and bottom of the cell (also called the basolateral membrane) is smooth. These two membranes have different jobs to do. The apical membrane faces inside the gut, and has the very important job of engulfing nutrients to transport through the cell, across the basolateral membrane, and then into the body.
What separates the apical and basolateral sides of the epithelial cell is a phenomenally complex and essential structure called a tight junction. Tight junctions are formed by the interweaving of dozens of different proteins, including several that cross the cell membrane (so part of the protein is inside the cell, part is embedded in the membrane, and part is outside the cell). These proteins tangle together in complex and dynamic ways to form a bond to the adjacent cells. Not only are tight junctions how the epithelial cell differentiates between its top and bottom, but it’s also part of the glue that holds this ever important sheet of cells together!
Tight junctions are dynamic structures and the epithelial cell can control just how open or closed the junction is. The junction can be rapidly opened and closed to allow some larger nutrients across the cell. But, when something happens to damage the tight junction, or impact the cell’s ability to control how open or closed it is, that’s when a variety of bad things happen.
 There are two ways that a gut can become “leaky”. If something damages the gut epithelial cell and the cell dies, that leaves a small hole (at least until the gut can repair itself). If something triggers the opening of the tight junctions, that also leaves small holes that substances inside the gut can leak through into the body. Not only does this create a pathway for things to leak into the body, but it’s also a tremendous stress on the cell. It can kill the cell (creating an even bigger hole), or worse: the loss of tight junction assembly is one of the steps for an epithelial cell to become a cancer cell.
There are two ways that a gut can become “leaky”. If something damages the gut epithelial cell and the cell dies, that leaves a small hole (at least until the gut can repair itself). If something triggers the opening of the tight junctions, that also leaves small holes that substances inside the gut can leak through into the body. Not only does this create a pathway for things to leak into the body, but it’s also a tremendous stress on the cell. It can kill the cell (creating an even bigger hole), or worse: the loss of tight junction assembly is one of the steps for an epithelial cell to become a cancer cell.
A leaky gut, or more technically “increased intestinal permeability”, means things can get across the gut barrier that aren’t supposed to. This happens when either the epithelial cells or the tight junctions are damaged. And right on the other side of that barrier is 80% of our body’s immune systems, acting as a sentinel, ready to attack anything that might try to cross the barrier. So, when you have a leaky gut, you also have an activated immune system. Read more in What Is A Leaky Gut? (And How Can It Cause So Many Health Issues?), What Should You Eat To Heal a Leaky Gut?, and 8 Nutrients for Leaky Gut.
Okay, now on to the how prolamins interact with our enterocytes…
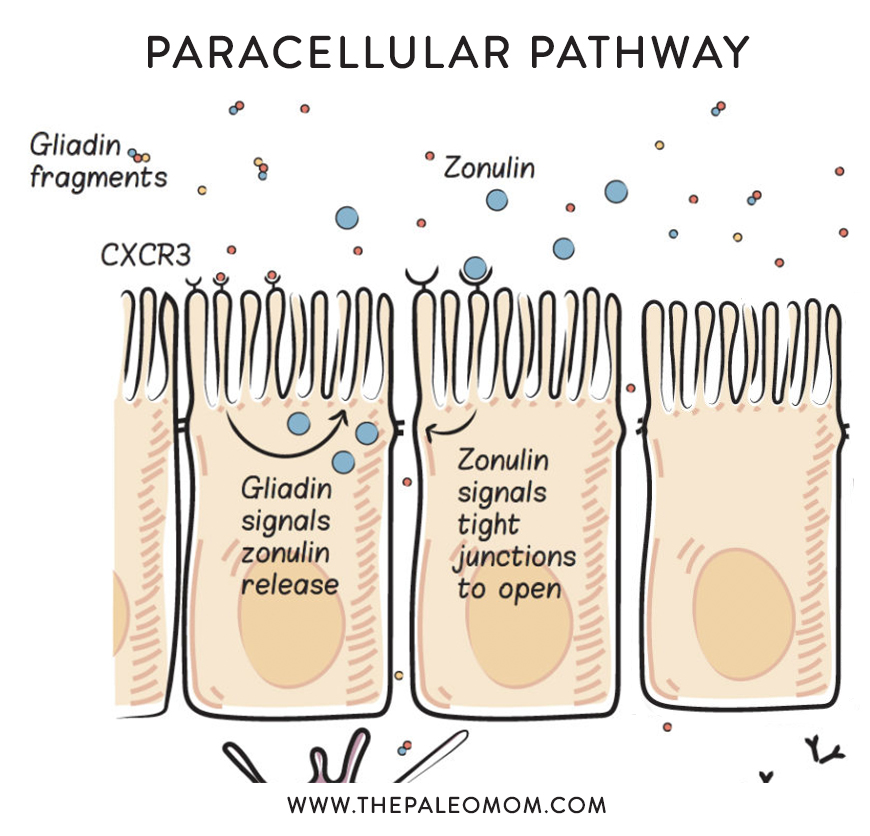
Paracellular Pathway – Zonulin
The best-understood way that gluten increases intestinal permeability is by stimulating the release of a protein called zonulin. Zonulin (also identified as haptoglobin 2 precursor) used to be thought of simply as a precursor protein, without a distinct function. However, now it is recognized as the only (so far, anyway) extracellular protein that impacts tight junction assembly. (As an aside, zonulin is a precursor for hatoblogin 2, which circulates in the blood and binds with free plasma hemoglobin to facilitate degradation and iron recycling while avoiding kidney damage from free hemoglobin.)
Zonulin is released by enterocytes into the gut lumen (inside the gut), where it travels downstream and then binds to at least two different types of receptors in the enterocyte apical membrane, PAR2 and EGFr, which creates intracellular signaling cascades that displace important tight junction proteins (decreasing the protein-protein interactions that form the tight junction) thereby causing tight junctions to open and intestinal permeability to increase.
Zonulin may play a normal role in digestion, by opening up the tight junctions to facilitate the absorption of some nutrients into the body. For example, even though glucose is actively transported into enterocytes (via the insulin-dependent GLUT4 transporter), the major portion of glucose is absorbed paracellularly, via increased tight junction permeability.
Zonulin may have a normal role in protecting us from gut pathogens. Zonulin secretion is also stimulated by the presence of enteroinfectious bacteria, which has two effects. First, when the tight junctions open due to zonulin secretion, the hydrostatic pressure gradient flips so that water is secreted into the intestinal lumen (as opposed to being absorbed into the body), which helps to flush bacteria out of the body (in fact, this may explain the high frequency of loose stools and/or diarrhea as a symptom of enteropathogens). Second the increased intestinal permeability exposes the invading pathogen to the immune system, allowing for a more efficient immune response. Interestingly, this might be why gut pathogens are common triggering events for autoimmune disease. And, here’s another way that an unhealthy or unbalanced gut microbiome may be linked to chronic disease: even nonpathogenic strains of E. coli (a Gram-negative normal resident of the human gut, at least in small quantities), causes an increase in intestinal permeability via increased zonulin release. This may represent another important link between leaky gut and the microbiome, see What Is the Gut Microbiome? And Why Should We Care About It?.
The problem is when there’s too much zonulin.
Too much zonulin causes increased intestinal permeability–yes, leaky gut. And, as you know, leaky gut is linked to every chronic illness in which a potential link has been investigated, hypothesized to be a necessary precursor to autoimmune disease and other conditions. Read more in What Is A Leaky Gut? (And How Can It Cause So Many Health Issues?), What Should You Eat To Heal a Leaky Gut?, and 8 Nutrients for Leaky Gut.
When we consume gluten, it is partially digested (because of its incompatibility with our digestive enzymes) and predictable gliadin fragments are produced. These gliadin fragments bind with a specific receptor in the apical cell membrane of enterocytes, called the chemokine receptor 3 (CXCR3) , which stimulates the release of zonulin into the gut lumen (inside the gut). This happens in everyone, but people with celiac disease are known to have an exaggerated zonulin response to gluten consumption, and this may be a key driver of the disease. Interestingly, even people without celiac disease but who still have celiac risk genes (HLA-DQ2 and HLA-DQ8, which impacts an estimated 40% to 55% of the population, see Genes to Know About: Celiac Genes) have an exaggerated zonulin response when they consume gluten. This may be the cause of Irritable Bowel Syndrome, but also increases risk of a variety of other chronic health problems. See also Why Grains Are Bad – Part 1, Lectins and the Gut, and Gluten Cross-Reactivity: How your body can still think you’re eating gluten even after giving it up.
In fact, increased zonulin levels are also linked to a variety of diseases, including:
- Celiac Disease
- Type-1-Diabetes
- Inflammatory Bowel Disease/Colitis
- Multiple sclerosis / EAE
- Obesity/Insulin resistance
- Type-2-Diabetes
- Polycystic ovary syndrome
- Acute Lung Injury
- Asthma
- Coronary artery disease
- Glioma
- Septicemia
- HIV
- Irritable Bowel Syndrome
- Non-Celiac gluten sensitivity
- Environmental Enteropathy
- Necrotizing Enterocolitis
A recent paper even linked low zonulin and endotoxin levels to longevity–centenarians have much lower zonulin levels than people aged less than 40 who had suffered acute myocardial infection and even lower than healthy young controls! Given that endotoxin is a toxic bacterial protein from the cell membranes of Gram-negative bacteria (which as discussed above, at least some of which stimulate zonulin release), it’s a reasonable assumption that being gluten-free and taking care of our gut microbiomes may be key to extending lifespan and lowering risk of chronic disease!
And that’s just one mechanisms by which gluten and other prolamins increase intestinal permeability!
Transcellular Pathways
There are two transcellular pathways—retrotranscytosis and lysosomal. Retrotranscytosis of gliadin has only recently been identified, and thus far has been studied only in the context of celiac disease. The mechanisms of retrotrancytosis are not necessarily limited to celiac disease, however, and could occur in anyone with gluten intolerance and iron deficiency. Lysosomal pathways are also not limited to those with gluten antibodies or genetic susceptibilities.
IgA antibodies produced by B cells in gut-associated lymphoid tissue are normally transported from the basolateral side of the gut enterocytes (the side facing inside the body) to the apical side of the gut enterocytes (the side facing inside the gut) and into the gut lumen (the space inside the gut). This is called transcytosis (which basically means transportation across a cell). IgA antibodies perform a variety of functions in the brush border and lumen of the intestine, including what is called immune exclusion, which is the interference with the ability of antigens (including viruses, bacteria, bacterial toxins, and enzymes) to adhere to and penetrate the gut barrier. IgA antibodies are then recycled by a mechanism called retrotranscytosis; that is, they are transported from the apical to the basolateral side of the cell, or from inside the gut back into the body. In addition to allowing for the recycling of these IgA antibodies, retrotranscytosis allows for antigen presentation from inside the gut to the immune system in a very controlled way. Importantly, retrotranscytosis may protect the gut enterocytes from viral and bacterial infection (by binding to antigens inside the cells) and thereby preserve the integrity of the gut barrier. Also, IgA retrotranscytosis is a form of protected transport across the cell, meaning that the IgA antibodies are not degraded or modified as they cross the cell (which occurs with many other forms of transport across the cell).
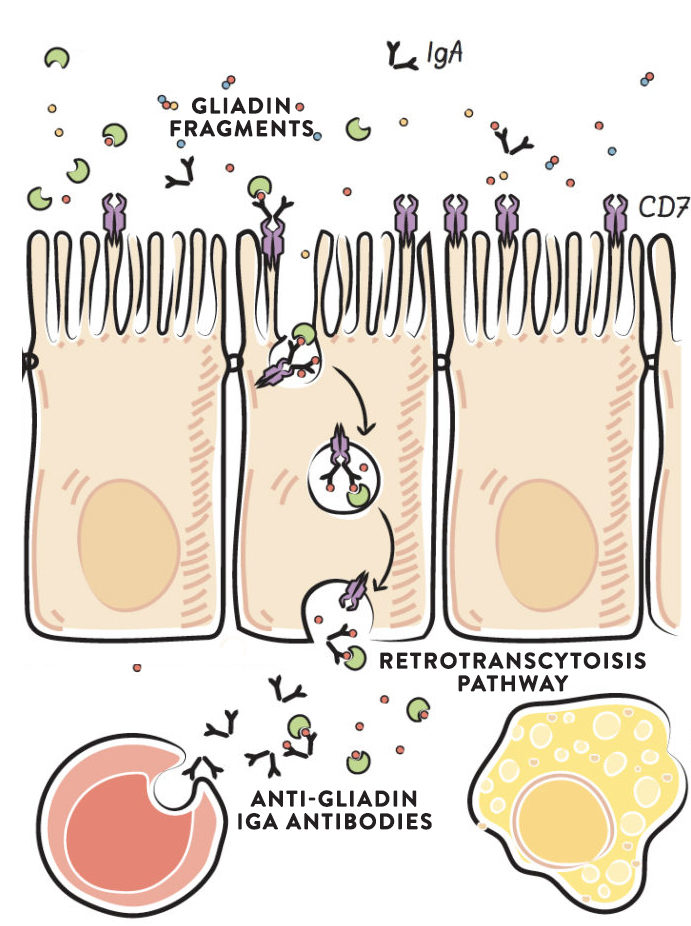 IgA antibodies produced against gliadin bind to specific gliadin fragments in the intestinal lumen and form a stable complex. This IgA-gliadin complex fits into a specific receptor (called the transferrin receptor, which is normally used for the absorption of iron) in the apical membrane of the gut enterocytes. The complex is retrotranscytosed, and this causes intact gliadin fragments to be delivered to the basolateral side of the cell, where they can activate the immune system. This retrotranscytosis of gliadin has been well-characterized in the context of celiac disease. Once across the cell, the gliadin fragments are delivered to the gut-associated lymphoid tissue to stimulate the innate and adaptive immune systems. Again, the resulting production of cytokines and stimulation of inflammation can cause damage to gut enterocytes, thereby causing a leaky gut.
IgA antibodies produced against gliadin bind to specific gliadin fragments in the intestinal lumen and form a stable complex. This IgA-gliadin complex fits into a specific receptor (called the transferrin receptor, which is normally used for the absorption of iron) in the apical membrane of the gut enterocytes. The complex is retrotranscytosed, and this causes intact gliadin fragments to be delivered to the basolateral side of the cell, where they can activate the immune system. This retrotranscytosis of gliadin has been well-characterized in the context of celiac disease. Once across the cell, the gliadin fragments are delivered to the gut-associated lymphoid tissue to stimulate the innate and adaptive immune systems. Again, the resulting production of cytokines and stimulation of inflammation can cause damage to gut enterocytes, thereby causing a leaky gut.
Where do these IgA antibodies against gliadin come from? No one knows, but high levels of these gliadin-specific serum IgA antibodies in the intestinal lumen are found not only in individuals with active celiac disease, but also in healthy individuals. Importantly, there are an abnormally high number of transferrin receptors, which transport the IgA-gliadin complex into the cell, in the gut enterocytes of celiac disease patients. The increase in transferrin receptors may be caused by iron deficiency (iron-deficiency anemia is extremely common in celiac disease), forming yet another link to micronutrient deficiency.
When gliadin crosses the gut barrier by retrotranscytosis, it is basically exploiting a normal recycling pathway and mechanism meant to protect the cells of the gut barrier from being damaged by infectious organisms.
Lysosomal Pathway
Even in healthy individuals, gliadin fragments are taken up by enterocytes through a process called endocytosis. Endocytosis is a normal function of all cells in the body by which they absorb molecules (such as long proteins that cannot enter the cells through the cell membrane or through specific transporters embedded in the cell membrane) by engulfing them (and other compounds) in a membranous structure (sort of like a bubble where the bubble surface is made of membrane that was part of the outer cell membrane before endocytosis). These “bubbles”are called endosomes, and they allow the cell to sort and recycle proteins in a targeted way (protein recycling is a very important function for every cell since it allows proteins to be reused, which is more efficient that building new proteins).
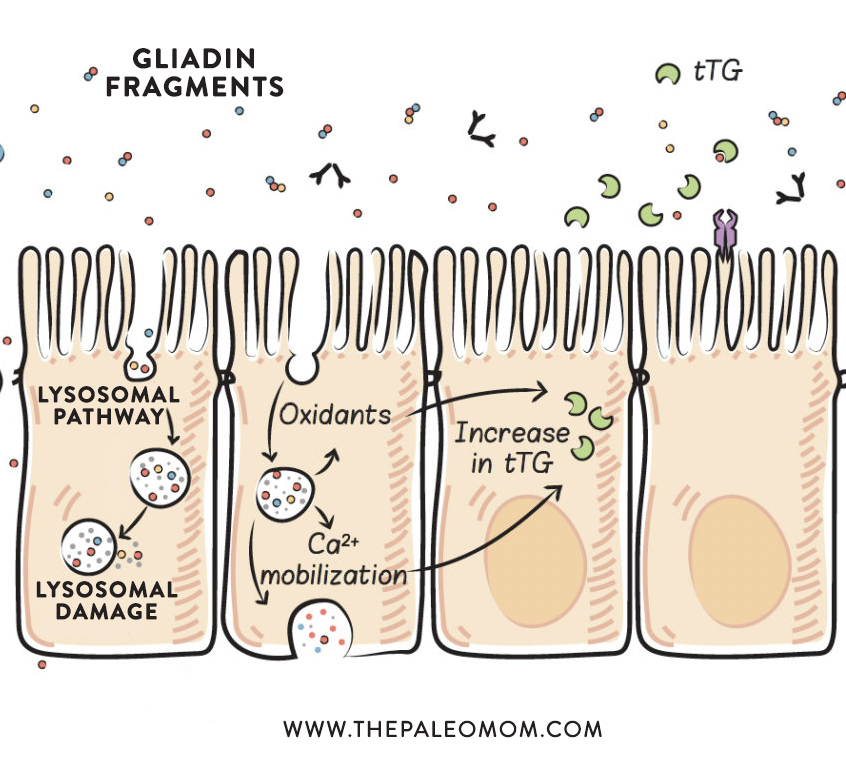 Within the enterocyte, endocytosed proteins are held within a type of endosome called a lysosome. Lysosomes contain enzymes (called lysosomal-acid proteases) that can break proteins apart into individual amino acids. Lysosomes then travel to the basolateral membrane (the side of the cell facing inside the body, rather than inside the gut), where the contents of the lysosome can be exocytosed (which is the opposite of endocytosis, in which the contents are secreted out of the cell and the lysosome membrane can integrate back into the outer cell membrane). Even though the proteins are traveling from the apical side of the cell to the basolateral side of the cell, this is transcytosis (because this is the normal direction of transport across the cell for these proteins). In many healthy individuals, gliadin peptides can be fully digested within the lysosomes, but this is not the case for those with celiac disease. It is unknown what percentage of gliadin peptides may remain undigested through this process in those with autoimmune disease.
Within the enterocyte, endocytosed proteins are held within a type of endosome called a lysosome. Lysosomes contain enzymes (called lysosomal-acid proteases) that can break proteins apart into individual amino acids. Lysosomes then travel to the basolateral membrane (the side of the cell facing inside the body, rather than inside the gut), where the contents of the lysosome can be exocytosed (which is the opposite of endocytosis, in which the contents are secreted out of the cell and the lysosome membrane can integrate back into the outer cell membrane). Even though the proteins are traveling from the apical side of the cell to the basolateral side of the cell, this is transcytosis (because this is the normal direction of transport across the cell for these proteins). In many healthy individuals, gliadin peptides can be fully digested within the lysosomes, but this is not the case for those with celiac disease. It is unknown what percentage of gliadin peptides may remain undigested through this process in those with autoimmune disease.
There is also evidence that lysosomal damage may occur in response to gliadin fragments. (Interestingly, casein, a milk protein, does the same thing.) If a lysosome is damaged, not only do the still-intact gliadin fragments enter the cell cytoplasm, but so do the enzymes within the lysosome, which can then attack proteins within the cell, damaging and probably killing the cell. Basically, if a lysosome is damaged while digesting and transporting proteins across the cell, the release of the contents of the lysosome within the cell causes the cell to die. A damaged or dead cell opens up a hole through which other components of the gut can leak into the body and activate the immune system. This is one mechanism by which gliadin (and casein) can cause a leaky gut, even in healthy individuals who have no gluten sensitivity or intolerance. It is unknown if a dietary threshold exists for this lysosomal damage to occur or whether such a threshold could vary depending on genetic or other factors.
An additional effect of the lysosomal pathway is the stimulation of inflammation through the production of oxidants. The accumulation of specific gliadin fragments within the lysosomes causes an increase in the production of reactive oxygen species (oxidants) without causing damage to the lysosome. Although not all the details of this process are understood, some of the signaling pathways have been uncovered, and it appears that some gliadin fragments stimulate signals known to drive inflammation. The production of oxidants can also cause damage to the cell, which may result in alterations in cell morphology (that is, cell shape, which also affects cell function) and cell division (proliferation), and may affect cell viability and cause apoptosis (programmed cell suicide). And, again, damage or enterocyte cell death leaves a hole in the gut barrier.
Another damaging effect of lysosomal transport of gliadin fragments is the mobilization of intracellular calcium-ion stores, which causes endoplasmic-reticulum stress (because the endoplasmic reticulum contains the highest concentration of calcium ions within the cell, and the cell cannot function properly if it loses them). The endoplasmic reticulum is the organelle within every cell responsible for protein synthesis, lipid metabolism, carbohydrate metabolism, and detoxification. When it is stressed, it can’t do its job efficiently. Calcium ions are mobilized in response to gliadin fragments in the intact lysosome, although we don’t yet know quite how. In the context of celiac disease, this production of oxidants and the mobilization of calcium ions drive the initial increase in tissue transglutaminase (see Why Grains Are Bad-Part 1, Lectins and the Gut). And importantly, when endoplasmic-reticulum stress is severe or prolonged, cell death (via apoptosis) occurs. Again, this causes a hole in the gut barrier. As in the case of lysosomal damage, it is unknown whether dietary thresholds or genetic predisposition are factors; however, both of these mechanisms appear to apply generally. The generation of reactive oxygen species and calcium-ion mobilization may also be the result of specific gliadin fragments entering the cell via retrotranscytosis.
What Is the Role of Genetics?
What’s the takeaway here? There are many ways the prolamin gliadin (the protein fraction of gluten) can cross and damage the gut barrier, even in people without a genetic susceptibility to a leaky gut and even in people without diagnosed gluten sensitivity. Whether by paracellular or transcellular pathways, once outside the gut, these protein fragments interact with the gut-associated lymphoid tissue, stimulating the release of inflammatory cytokines and activating cells of both the innate and adaptive immune systems.
 There are a variety of ways gliadin can cause damage and death of gut enterocytes, all of which result in holes in the gut barrier through which various contents of the gut can leak out. Inflammation is triggered by gliadin fragments that cross the gut barrier, as well as by other partly digested food proteins, gut bacteria, bacterial fragments, and waste products or toxins likewise crossing over. This further activates the immune system, causing a vicious cycle of inflammation and gut-barrier damage.
There are a variety of ways gliadin can cause damage and death of gut enterocytes, all of which result in holes in the gut barrier through which various contents of the gut can leak out. Inflammation is triggered by gliadin fragments that cross the gut barrier, as well as by other partly digested food proteins, gut bacteria, bacterial fragments, and waste products or toxins likewise crossing over. This further activates the immune system, causing a vicious cycle of inflammation and gut-barrier damage.
How important is genetic susceptibility? Certainly it is involved in the zonulin response to gliadin (it is unknown if HLA-DQ2 and HLA-DQ8 are the only genes that impact zonulin secretion). Although the role that genetic factors play in enterocyte damage caused by transport through the gut enterocytes remains unstudied, genetic predisposition may explain the variability in the severity of the damage caused by wheat consumption. It is likely that some damage is caused to the gut barrier of everyone who consumes wheat, but genes might explain why some people suffer from a severely leaky gut or autoimmune disease in response to wheat consumption while others seem to tolerate wheat without experiencing any overt health issues.
It is important to emphasize that studies do confirm that gliadin fragments enter the human body intact, and bind with receptors in human cells, indicating biological activity. This is particularly implicated in obesity, see The Link Between Gluten and Obesity
Prolamins and Gut Bacteria
Prolamins also contribute to gut dysbiosis, which, as discussed What Is the Gut Microbiome? And Why Should We Care About It?, can cause a leaky gut all by itself. Not only are prolamins inherently difficult for our bodies to digest, but they interfere with important digestive enzymes in the brush border of the intestine. In particular, gliadin is known to inhibit the activity of three important enzymes: lactase, sucrase, and dipeptidyl peptidase 4. These are important for breaking sugars down into monosaccharides and proteins into amino acids for transport across the enterocyte barrier (lactase breaks apart lactose; sucrase breaks apart sucrose; dipeptidyl peptidase 4 has diverse functions related to digestion, metabolism, and immune regulation). This may have a profound effect on the gut microbiome because the inhibition of these enzymes alters what’s available for gut bacteria farther down the digestive tract (see Modifying Paleo for Small Intestinal Bacterial Overgrowth (SIBO))
A recent study in gluten-sensitive primates demonstrated that gluten consumption causes a substantial and undesirable shift in the microbiome. In particular, gluten consumption significantly decreased alpha-diversity of the microbiome (recall that diversity is the single most important train of a healthy microbiome) with certain bacterial families being enriched (e.g., Streptococcaceae and Lactobacillaceae).. A recent human study evaluating the microbiome shift caused by adopting a gluten-free diet showed decreases in several bacteria species, the strongest effect being a substantial reduction in Veillonellaceae, a Gram-negative, pro-inflammatory bacterium linked to Crohn’s disease and other gut pathologies.
Is This Just about Gluten?
So, if most of what is known about prolamins comes from the study of gluten and celiac disease, how do we know this applies to all prolamins?
There is much similarity in structure and function (due to homology, or similarity in amino acid sequences) between the prolamins of different grains and legumes. While there have been no comprehensive studies evaluating the detrimental health effects of all food sources of prolamins, there is enough convincing evidence of similar proteins in nongluten-containing grains and pseudo-grains to support their complete removal from our diets. For example, studies show that prolamins in quinoa, corn, and oats can cause damage to the gut and stimulate the immune system in celiac sufferers in a manner completely analogous to gliadin. Clearly, this means that those with celiac disease should never consume these other grains or pseudo-grains. But also, because of the understanding that gluten increases intestinal permeability, which is strongly linked with all chronic illness, anyone struggling with a diagnosis should probably avoid the consumption of grains, pseudo-grains, and legumes.
Citations
Alaedini, A. & Latov, N., Transglutaminase-independent binding of gliadin to intestinal brush border membrane and GM1 ganglioside, J Neuroimmunol. 2006 Aug;177(1-2):167-72
Ballard ST, et al. Regulation of tight-junction permeability during nutrient absorption across the intestinal epithelium. Annu Rev Nutr. 1995;15:35-55.
Bonder MJ, et al. The influence of a short-term gluten-free diet on the human gut microbiome. Genome Med. 2016 Apr 21;8(1):45. doi: 10.1186/s13073-016-0295-y.
Cabrera-Chávez, F., et al., Maize prolamins resistant to peptic-tryptic digestion maintain immune-recognition by IgA from some celiac disease patients, Plant Foods Hum Nutr. 2012 Mar;67(1):24, 30
Caputo, I., et al., Gliadin peptides induce tissue transglutaminase activation and ER-stress through Ca2+ mobilization in Caco-2 cells, PLoS One. 2012;7(9):e45209
Carrera-Bastos, P, et al. Serum Zonulin and Endotoxin Levels in Exceptional Longevity versus Precocious Myocardial Infarction. Aging Dis. 2018 Apr; 9(2): 317–321.
Clemente MG, et al. Early effects of gliadin on enterocyte intracellular signalling involved in intestinal barrier function. Gut. 2003;52:218–23.
de Rooij, F.W., et al., Lysosomal damage by gliadin and gliadin peptides; an activity not related to coeliac disease, Clin Chim Acta. 1979 Jan 15;91(2):127-31.
El Asmar R, et al. Host-dependent zonulin secretion causes the impairment of the small intestine barrier function after bacterial exposure. Gastroenterology. 2002;123:1607–15.
Fasano A. Leaky gut and autoimmune diseases. Clin Rev Allergy Immunol. 2012 Feb;42(1):71-8.
Fasano A. Zonulin and its regulation of intestinal barrier function: the biological door to inflammation, autoimmunity, and cancer. Physiol Rev. 2011 Jan;91(1):151-75.
Fasano A., Physiological, pathological, and therapeutic implications of zonulin-mediated intestinal barrier modulation: living life on the edge of the wall. Am J Pathol. 2008; 173:1243–1252
Fasano A., Surprises from celiac disease. Sci Am 2009; 301:54–61
Fasano, A., Zonulin, regulation of tight junctions, and autoimmune diseases, Ann N Y Acad Sci. 2012 Jul;1258:25-33
Friis, S., et al., Gliadin uptake in human enterocytes. Differences between coeliac patients in remission and control individuals, Gut. 1992 November; 33(11): 1487–1492
Heyman, M. & Menard, S., Pathways of gliadin transport in celiac disease, Ann N Y Acad Sci. 2009 May;1165:274-8
Holding, D. & Messing, J., Evolution, Structure, and Function of Prolamin Storage Proteins, in Seed Genomics (ed P. W. Becraft), 2013, Wiley-Blackwell, Oxford, UK.
Kozáková, H., et al., Brush border enzyme activities in the small intestine after long-term gliadin feeding in animal models of human coeliac disease, Folia Microbiol (Praha). 1998;43(5):497-500
Lebreton, C., et al., Interactions among secretory immunoglobulin A, CD71, and transglutaminase-2 affect permeability of intestinal epithelial cells to gliadin peptides, Gastroenterology. 2012 Sep;143(3):698-707.e1-4
Luciani, A., et al., Lysosomal accumulation of gliadin p31-43 peptide induces oxidative stress and tissue transglutaminase-mediated PPARgamma downregulation in intestinal epithelial cells and coeliac mucosa, Gut. 2010 Mar;59(3):311-9
Mamone, G., et al., Identification of a peptide from alpha-gliadin resistant to digestive enzymes: implications for celiac disease, J Chromatogr B Analyt Technol Biomed Life Sci. 2007 Aug 15;855(2):236-41
Matysiak-Budnik, T., et al., Alterations of the intestinal transport and processing of gliadin peptides in celiac disease, Gastroenterology. 2003 Sep;125(3):696-707
Matysiak-Budnik, T., et al., Secretory IgA mediates retrotranscytosis of intact gliadin peptides via the transferrin receptor in celiac disease, J Exp Med. 2008 January 21; 205(1): 143–154
Mazumdar, K., et al., Visualization of transepithelial passage of the immunogenic 33-residue peptide from alpha-2 gliadin in gluten-sensitive macaques, PLoS One. 2010 Apr 19;5(4):e10228
Ménard S., et al., Paracellular versus transcellular intestinal permeability to gliadin peptides in active celiac disease, Am J Pathol. 2012 Feb;180(2):608-15
Reichelt KL, Jensen D. IgA antibodies against gliadin and gluten in multiple sclerosis. Acta Neurol Scand. 2004 Oct;110(4):239-241.
Mohan M, et al. Dietary Gluten-Induced Gut Dysbiosis Is Accompanied by Selective Upregulation of microRNAs with Intestinal Tight Junction and Bacteria-Binding Motifs in Rhesus Macaque Model of Celiac Disease.Nutrients. 2016 Oct 28;8(11). pii: E684.
Sturgeon C, Fasano A. Zonulin, a regulator of epithelial and endothelial barrier functions, and its involvement in chronic inflammatory diseases. Tissue Barriers. 2016 Oct 21;4(4):e1251384.
Uibo, O., et al., Serum IgA anti-gliadin antibodies in an adult population sample. High prevalence without celiac disease, Dig. Dis. Sci. 1993; 38:2034–2037
van de Wal, Y., et al., Selective deamidation by tissue transglutaminase strongly enhances gliadin-specific T cell reactivity, J Immunol. 1998 Aug 15;161(4):1585-8
Vanuytsel T, et al, The role of Haptoglobin and its related protein, Zonulin, in inflammatory bowel disease. Tissue Barriers. 2013 Dec 1;1(5):e27321.
Vojdani, A. & Tarash, I., Cross–Reaction between Gliadin and Different Food and Tissue Antigens,Food and Nutrition Sciences 2013; 4 (1): 20-32
Zevallos, V.F., et al., Variable activation of immune response by quinoa (Chenopodium quinoa Willd.) prolamins in celiac disease, Am J Clin Nutr. 2012 Aug;96(2):337-44