SCIENCE SIMPLIFIED:

- A human study was published looking at how long-term adherence to a Paleo diet affects the gut microbiota and production of TMAO, which is linked to cardiovascular disease and some forms of cancer.
- The study demonstrates that a low-carb Paleo diet increases TMAO, attributable to a decrease in probiotic bacteria from the Bifidobacterium and Roseburia genera and an increase in Hungatella species in the gut.
- The dietary factors most strongly associated with the undesirable gut microbiome shift and TMAO production were total carbohydrate intake and resistant starch intake.
- While the authors conclude that lack of grains are to blame, the study participants were eating mostly non-starchy vegetables (about 7 servings per day, averaging 28 grams of fiber but only 90 grams of total carbohydrates). Starchy roots and tubers are a healthy alternative to grains and would have increased the participant’s total carbohydrate intake (all slow-burning nutrient-dense carbs) as well as resistant starch intake.
- The results of this study are relevant to our community and offer a stern warning against long-term low-carb implementations of the Paleo diet.
RELATED READING:
- What Is the Gut Microbiome? And Why Should We Care About It?
- The Case for More Carbs: Insulin’s Non-Metabolic Roles in the Human Body
- Why Root Veggies Are Great for the Gut Microbiome
Whenever a new Paleo study comes out, it’s sure to stir up controversy—no matter what the findings are. “Good” findings are met with cheers from the Paleo community, while “bad” findings tend to explode in media headlines and send the Paleo community rushing to find reasons the study is poorly conducted, inaccurate, or otherwise worth dismissing.
As you know, I don’t believe in dismissing scientific findings (except for the rare occasion where flaws in how the study was designed or conducted result in retraction). Instead, I seek to understand the data in the context of the research field as a whole, seeking context and nuance while being open to adjusting my conclusions and recommendations. (I discuss the merits of different types of scientific studies as well as the problem with cherry-picking data in my Introduction to Nutritional Sciences online course.)
Recently, a human study was published looking at how long-term adherence to a Paleo diet affects the gut microbiota and TMAO. And, the headlines (both mainstream media and outlets targeting physicians) have not been flattering, for example: “Paleo Diet Increases Risk for Heart Disease”, “Paleo Diet Study Links ‘Caveman’ Regime to Heart Disease Biomarker”, and “Study Linking Paleo Diet to Increased Heart Disease Risk Strengthens Diet Industry Concerns”.
As you’re probably aware by now, I’m all about the gut microbiome, and believe it will continue to prove itself as a massive missing piece in our health puzzle! For that reason, this study is particularly interesting and important to look at (and not dismiss!).
Before we dive into the study itself, let’s review the things we need to know to make sense of the findings!
A Refresher on Fiber, Resistant Starch, and the Microbiome
 The gut is a biological niche, home to a diverse array of microbes that influence nearly all aspects of human biology through their interactions with our bodies. We have a symbiotic relationship with these microbes, collectively referred to as our gut microbiome—in exchange for food and shelter, they contribute to our wellbeing by performing diverse functions essential to our health.
The gut is a biological niche, home to a diverse array of microbes that influence nearly all aspects of human biology through their interactions with our bodies. We have a symbiotic relationship with these microbes, collectively referred to as our gut microbiome—in exchange for food and shelter, they contribute to our wellbeing by performing diverse functions essential to our health.
When we don’t have a healthy diversity of the right kinds of microbes in our gut, our entire body suffers. An unfavorable gut microbiome has been linked to conditions as wide-ranging as cancer, obesity and other metabolic problems, heart disease, anxiety, depression, autism, autoimmunity, ulcers, IBD, liver disease, systemic infections, and more. See What Is the Gut Microbiome? And Why Should We Care About It?.
Nutrivore Weekly Serving Matrix
An easy-to-use and flexible weekly checklist
to help you maximize nutrient-density.
The Weekly Serving Matrix is very helpful! I’ve been eating along these lines but this really helps me know where to focus vs. which foods serve a more secondary role. It’s super helpful and has taken a lot of worry out of my meal planning. Thanks!
Jan
I’m writing a book on the gut microbiome, so I could expound for days, but let’s focus on a few specifics that are particularly important to understanding this new paper.
Fiber Is Food for Our Gut Microbiome
If there’s one thing our gut microbes love, it’s fiber. In fact, out of all the dietary factors that can impact the gut, fiber may be the most important.
Carbohydrates, including fiber, are chains of monosaccharides (simple sugars) and of chemical derivatives of monosaccharides. Both the types of simple sugar (and their derivatives) in the chain and the ways they link together to form chains (both overall structure and the types of chemical bonds between sugar molecules) determine what type of carbohydrate it is. What separates fiber from other carbohydrates is that the way the sugars link together are not compatible with our digestive enzymes; our bodies just aren’t capable of breaking apart those types of molecular bonds. Instead, fiber passes through the digestive tract mainly intact. And once it reaches the colon, the magic begins: fiber serves as a substrate (food) for a wide range of bacteria, including some of the most important species we can harbor!
Why Different Fiber Types Feed Different Bacteria
 The bacteria that live in our guts are collectively capable of producing over ten thousand different enzymes that can break down complex carbohydrates like fiber, which is pretty remarkable when you consider that we only produce about 17 different enzymes that digest carbohydrates! These enzymes belong to at least 206 different families of CAZymes (which stands for “Carbohydrate-Active enZymes”). Enzymes are typically highly specialized, breaking apart only specific molecular bonds, which is why different types of carbohydrates require different enzymes to break them apart (and why we can’t digest fiber but our gut bacteria can).
The bacteria that live in our guts are collectively capable of producing over ten thousand different enzymes that can break down complex carbohydrates like fiber, which is pretty remarkable when you consider that we only produce about 17 different enzymes that digest carbohydrates! These enzymes belong to at least 206 different families of CAZymes (which stands for “Carbohydrate-Active enZymes”). Enzymes are typically highly specialized, breaking apart only specific molecular bonds, which is why different types of carbohydrates require different enzymes to break them apart (and why we can’t digest fiber but our gut bacteria can).
When it comes to carbohydrate degradation, some bacteria are highly specialized, producing only a few dozen CAZymes, whereas others are multitaskers, producing hundreds of CAZymes which allows them to grow on a variety of substrates and adapt to changing nutritional circumstances, thereby giving them a competitive advantage in the gut ecosystem.
Bacteria from the Bacteroides genus in general tend to be multitaskers, producing enzymes that can break down starch, pectin, hemicelluloses, and other plant carbohydrates (galactomannan, arabinogalactan, alginate, laminarin and xylans xyloglucan, rhamnogalacturonans I and II, β-glucans and glucomannan, to name a few!). Bacteroides thetaiotaomicron is an example of multitasker, considered a diet insensitive strain thanks to its ability to produce around 400 CAZymes (at least 260 of which are glycoside hydrolases) that help it thrive on pectins as well as complex carbohydrates that are produced by our own intestinal cells, such as mucin (a glycoprotein that is a major component of mucus).
In contrast, Bifidobacterium species are more specialized, producing an average of 45 CAZymes that make them highly effective at degrading high amylose starches, including resistant starch, as well as fructooligosaccharides, galactooligosaccharides, and inulin fiber. The critical consequence is that Bifidobacterium don’t survive if our diet doesn’t include sufficient amounts of these starches and fiber types.
Importance of Fiber Variety in the Diet
One of the consequences of this diversity in CAZyme production by various resident microbes is that different fermentable carbohydrates support different species in the gut. Therefore, consuming a variety of dietary fibers—various cellulose, hemicellulose, pectins, gums, fructans, glucans, mucilage, chitin, chitosan, and resistant starch fiber—best supports a diverse microbial community compared to a diet comprised of less varied fermentable carbohydrates like the Standard American Diet which is rich in refined carbohydrates.
Which foods are rich in which fiber sources?
- Cellulose is found in all plants, but foods that contain particularly large amounts of cellulose include bran, legumes, nuts, peas, root vegetables, celery, broccoli, peppers, cabbage and other substantial leafy greens like collards, and apple skins. See The Fiber Manifesto-Part 2 of 5: The Many Types of Fiber
- Hemicellulose is particularly high in bran, nuts, legumes, and whole grains, as well as many green and leafy vegetables.
- Pectin is found in all fruits and vegetables but are particularly rich in certain fruits, including apples and citrus fruits, and are also found in legumes and nuts. See Why Fruit is a Good Source of Carbohydrates
- Lignin is most commonly a component of wood, but food sources include root vegetables, vegetable filaments (like the stems of leafy greens and the strings in celery), many green, leafy vegetables, wheat, and the edible seeds of fruit (such as berry seeds and kiwi seeds). See Polyphenols: Magic Bullet or Health Hype?
- Chitin is found not only in plants and fungi but also in the exoskeletons of insects and in the shells of crustaceans. See Elevating Mushrooms to Food Group Status and Why Crickets Are Great for the Gut Microbiome.
- Chitosan is naturally found in the cell walls of fungi but are also produced as a functional fiber by treating shrimp and other crustacean shells with sodium hydroxide. See Elevating Mushrooms to Food Group Status.
- Beta-glucans are found in some grains (mainly oats and barley, but also rye and wheat), fungi (yeast and mushrooms, particularly those mushrooms that are used medicinally like shiitake and maitake), and some types of seaweed (mainly algae). Beta-glucans are the fiber in oats that are largely responsible for their unique health benefits among grains. See Why Seaweed is Amazing! and Elevating Mushrooms to Food Group Status.
- Fructans are naturally occurring in a variety of plants and are particularly high in chicory, Jerusalem artichoke and alliums (the onion family). Cruciferous vegetables contain modest amounts of fructans. See What about the Goitrogens in Cruciferous Veggies?.
- Gums are a diverse group of fibers that plants secrete when they are damaged. Isolated versions are used in food manufacturing as thickening and gelling agents (like guar gum and xanthum gum). See Is It Paleo? Guar Gum, Xanthan Gum and Lecithin, Oh My!.
- Mucilages are particularly concentrated in cacti and other succulents (like aloe), many types of seaweed (like agar agar algae), flax, chia and psyllium. They can also be found in relatively large amounts in a variety of fruits and vegetables, including plantains, bananas, taro root, cassava, and berries. See Mucilaginous Fiber: The Good, the Bad, and the Gooey and The Verdict on Psyllium Husks: Not Paleo!.
- Resistant starch is divided into four subgroups: RS1 is rich in grains, legumes, and seeds; RS2 is found in most roots and tubers, and particularly high in green bananas, green plantains, and raw potatoes; RS3 is rich in cooked and cooled roots and tubers, particularly potatoes, as well as cooked and cooled rice; RS4 is the product of enzymatically or chemically modified starches sold under various brand names. See also Resistant Starch: It’s Not All Sunshine and Roses.
Because of their varying fiber types (and other food compounds that influence gut microbial composition like micronutrients, phytochemicals, proteins and fats), different families of fruits and vegetables are independently beneficial for the gut microbiome. See also The Fiber Manifesto-Part 2 of 5: The Many Types of Fiber
That means that beyond aiming for 8+ servings of fresh vegetables and fruits daily (see also The Importance of Vegetables), it’s important to hit as many of the different families of vegetables and fruits each day, ideally cycling through all of them every two days: cruciferous vegetables, leafy vegetables, roots and tubers, mushrooms, alliums, apple family, citrus, berries, nuts and seeds, etc.
Resistant Starch-Rich Foods as Microbiome Superfoods
Resistant starch is classified as a fiber because amylase, the enzyme that breaks starch into individual glucose units, doesn’t work on this type of starch.
Resistant starch is insoluble yet highly fermentable, and is divided into four subgroups: RS1, which is physically inaccessible due to being bound within cell walls; RS2, which is tightly packed ungelatinized granules found in certain raw starchy foods; RS3 (retrograded amylose), which is formed when certain starchy foods are cooked and then cooled down; and RS4, which is formed via a manmade chemical process. See also Resistant Starch: It’s Not All Sunshine and Roses
In general, resistant starch is famous for feeding short-chain-fatty-acid-producing bacteria and enhancing levels of butyric acid. In a human study, RS2 was shown to increase the abundance of Ruminococcus bromii and Eubacterium rectale, whereas RS4 increased Bifidobacterium adolescentis and Parabacteroides distasonis. Long-term feeding of RS1 and RS2 in rats showed that both types increased the abundance of anaerobes, the levels of Bifidobacteria, and total SCFAs in the cecum; meanwhile, RS2 (but not RS1) enhanced levels of Lactobacillus, Streptococci, and Enterobacteria. In pigs, RS3 has been shown to increase the abundance of Faecalibacterium prausnitzii while reducing levels of E. coli and Pseudomonas species. Interestingly, Ruminococcus bromii plays a keystone role in the degradation of resistant starch, releasing breakdown products that are then utilized by other microbes in the gut.
Two Important Probiotic Bacteria: Bifidobacteria and Roseburia
 Hundreds of beneficial probiotic species of bacteria (as well as fungi like yeast and archaea, which we’ll come back to) have been well studied in terms of their roles in the gut microbiome and human health in general. I want to focus in on two genera of bacteria that are particularly relevant to this new paper.
Hundreds of beneficial probiotic species of bacteria (as well as fungi like yeast and archaea, which we’ll come back to) have been well studied in terms of their roles in the gut microbiome and human health in general. I want to focus in on two genera of bacteria that are particularly relevant to this new paper.
The Bifidobacteria genus exerts a range of beneficial effects on human health, including producing vitamins, inhibiting pathogens from colonizing or infecting the gut mucosa, helping regulate the microbiome’s homeostasis, modulating local and systemic immune responses, repressing potentially carcinogenic enzymatic activities among different bacteria, and facilitating the bioconversion of various dietary compounds into bioactive forms. In a variety of studies, Bifidobacteria have been shown to improve the gut barrier function, suppress E. coli, improve glucose tolerance, reduce low-grade inflammation, and reduce endotoxemia induced by high-fat diets. As another benefit, because Bifidobacteria produce lactic acid instead of gas, people with higher levels tend to have less flatulence and digestive problems!
There are 39 identified species of Bifidobacteria, which represent 3-6% of health adult fecal flora. Their favorite foods are resistant starch 2, resistant starch 4, oligosaccharides from plants and milk, and hexose.
Let me emphasize: Bifidobacterium are critically important to human health. If you see an argument dismissing this paper because somehow low-carb diets or low-carb Paleo benefits make up for missing Bifidobacteria, that is unequivocally false.
The Roseburia genus are butyrate-producing bacteria that are implicated in maintaining gut barrier health and immune regulation, with strong anti-inflammatory properties. Reduced levels of Roseburia are associated with inflammatory bowel disease (Roseburia actually suppress the pathogenesis of Crohn’s disease), irritable bowel syndrome, obesity, type 2 diabetes, cardiovascular disease, neurological diseases, autoimmune disease, asthma and allergies—likely mediated through higher intestinal permeability and inflammation.
There are five known species of Roseburia (R. intestinalis, R. hominis, R. inulinivorans, R. faecis and R. cecicola) and their favorite foods are beta-glucans, fructans, pectin and resistant starch.
The Deal with TMAO
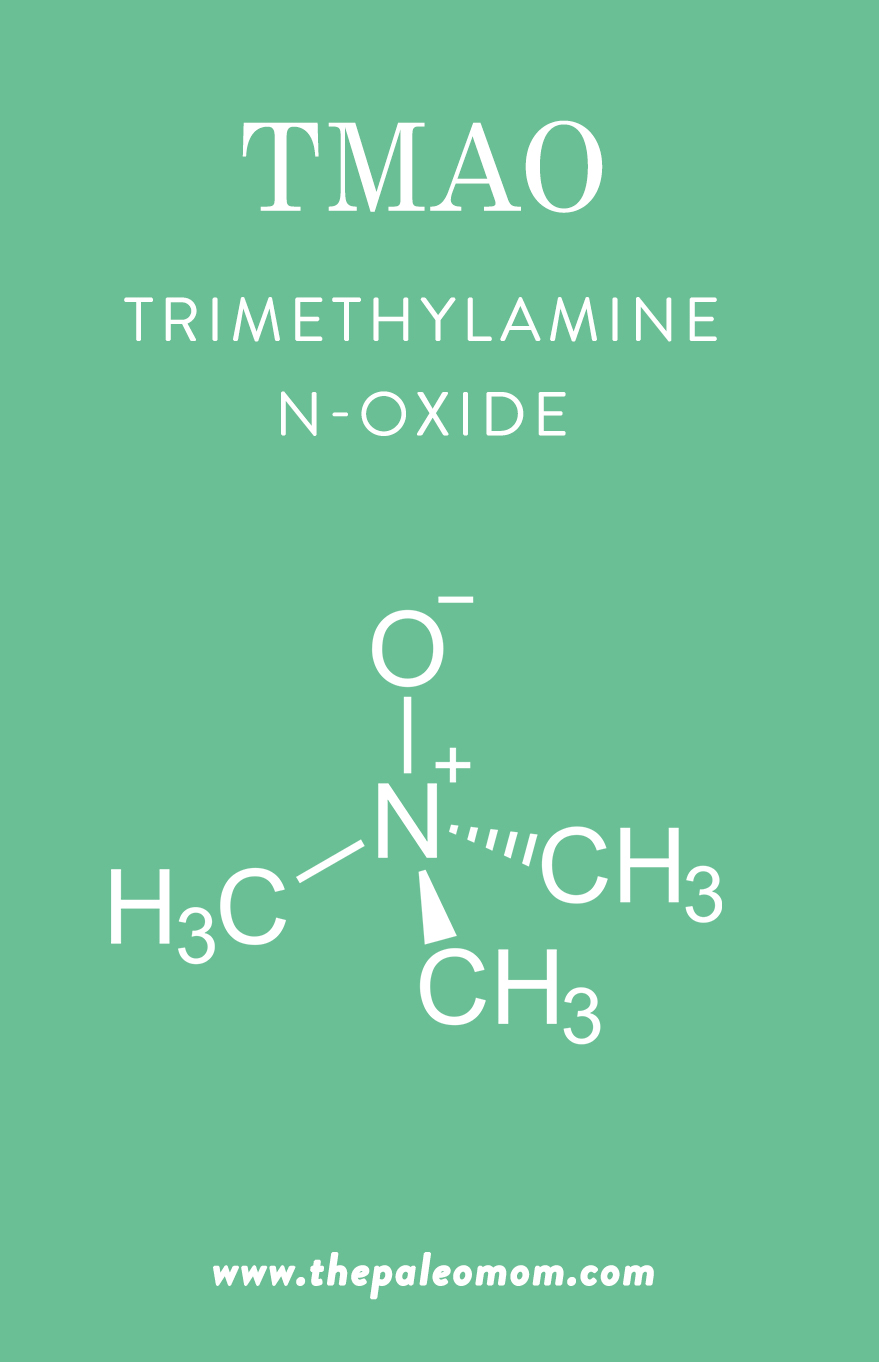 So, where does TMAO fit into the microbiome?
So, where does TMAO fit into the microbiome?
In recent years, trimethylamine-N-oxide (TMAO) has emerged as a possible new risk factor for cardiovascular disease, with potential to other conditions as well (such as chronic kidney disease, chronic heart failure, and colorectal cancer). For instance, a meta-analysis using 11 prospective studies found that higher circulating levels of TMAO were associated with a 23% increased risk of cardiovascular events and a 55% increased risk of all-cause mortality. This little molecule gets produced from the microbial metabolism of choline, lecithin, and carnitine (from our diet) into trimethylamine (TMA), which the liver then oxidizes into TMAO. Once it’s in our bodies, TMAO gets transported to different tissues and can potentially accumulate.
However, our levels of TMAO aren’t just a matter of how much free TMAO or TMAO precursors we ingest! Gut bacteria, especially the genus Prevotella, is a key mediator between diet and the amount of TMAO in our blood. In one study, researchers found that participants with gut microbiomes dominated by Prevotella were the ones who produced the most TMA (and therefore TMAO, after it reached the liver) from the carnitine they consumed. Those with microbiomes high in Bacteroides rather than Prevotella saw dramatically less conversion to TMA and TMAO.
So far, the list of TMAO-producing bacteria also includes multiple Clostridium species, Desulfovibrio desulfuricans, Providencia rettgeri, Edwardsiella targa, Escherichia fergusonii, Anaerococcus hydrogenalis, Proteus penneri, Firmicutes, and Proteobacteria. In other words, we can’t look at TMAO without also looking at the composition of our gut microbiota!
TMAO: Symptom or Cause of Heart Disease?
Despite some compelling associations with disease and mortality, there’s still a lot of controversy over whether TMAO has a causal relationship with the conditions it’s been associated with. On one hand, we have identified some plausible mechanisms for its role in disease, especially heart disease. For example, TMAO appears to increase the number of scavenger receptors in macrophages, resulting in greater binding to LDL particles and subsequent foam cell formation (foam cells play a major role in the progression of atherosclerotic plaque buildup!). TMAO also interferes with sterol transportation and bile acid metabolism, which can further contribute to the development of heart disease. And, TMAO may increase platelet activity, which can raise the risk of thrombosis by promoting excessive coagulation in the blood.
But despite these possible mechanisms, the literature is still mixed when it comes to how elevated TMAO plays out in real life! Along with studies showing a link between high TMAO levels and disease or mortality, many studies have failed to find any predictive value for TMAO on cardiovascular events, chronic kidney disease, or heart failure.
It’s possible that high TMAO occurs as a result of some of these diseases, rather than as a causative factor (high TMAO makes sense in the case of kidney failure, for instance, because the kidneys are the major site of circulating TMAO clearance, and their compromised function would naturally lead to a buildup of TMAO).
It’s also very possible that gut microbiota compositions that contribute to chronic disease also happen to result in more TMA and TMAO production, making TMAO a marker for disease states rather than a causal risk factor. Overall, the jury’s still out!
The Fish Paradox
 One of the major monkey wrenches in the whole TMAO story is a fishy one (pun intended). Seafood—famously considered heart-healthy—is one of the most abundant sources of free TMAO, and also contains TMAO precursors. And we’d be hard pressed to find a study that shows seafood is bad for our cardiovascular health (indeed, the vast majority of research shows that fish is one of the best things we can eat for our hearts!). Physiologically, TMAO helps fish survive in their marine environments by increasing buoyancy, acting as a form of antifreeze (by increasing osmotic concentration), and protecting tissue proteins against destabilizing forces. The degradation of TMAO into TMA is also what gives seafood its characteristic fishy odor! TMAO levels can vary dramatically between different species of fish (and even vary within the same species depending on the season), but in general, deep-sea fish and shellfish tend to be higher in TMAO than shallow water fish and shellfish, likely because of the role TMAO plays in protecting against pressure-induced protein damage.
One of the major monkey wrenches in the whole TMAO story is a fishy one (pun intended). Seafood—famously considered heart-healthy—is one of the most abundant sources of free TMAO, and also contains TMAO precursors. And we’d be hard pressed to find a study that shows seafood is bad for our cardiovascular health (indeed, the vast majority of research shows that fish is one of the best things we can eat for our hearts!). Physiologically, TMAO helps fish survive in their marine environments by increasing buoyancy, acting as a form of antifreeze (by increasing osmotic concentration), and protecting tissue proteins against destabilizing forces. The degradation of TMAO into TMA is also what gives seafood its characteristic fishy odor! TMAO levels can vary dramatically between different species of fish (and even vary within the same species depending on the season), but in general, deep-sea fish and shellfish tend to be higher in TMAO than shallow water fish and shellfish, likely because of the role TMAO plays in protecting against pressure-induced protein damage.
Research has shown that following the consumption of seafood, blood levels of TMAO levels rise to significantly higher levels than after the consumption of beef or eggs. In one study, 40 participants were fed meals containing cod fish, eggs, beef, or a fruit control in random order, with week-long washout periods between each intervention. After the fish meal, participants’ plasma TMAO rose up to 62 times higher than after the beef, eggs, or fruit meals. These levels peaked at 2 hours post-meal and remained elevated for the remainder of the 6-hour study period. The rise in TMAO levels started within 15 minutes following fish consumption, indicating the TMAO was being directly absorbed rather than undergoing conversion in the gut by bacteria. This same study found dramatic variations in TMAO response after eating eggs or beef. Compared to baseline, TMAO levels ranged anywhere from a 30% decrease to a 270% increase!
Food vs. Microbiome Sources of TMAO
So, why would we see such extreme variations in TMAO levels after eating the same foods? The answer is, not surprisingly, bacteria! Stool analysis showed that compared to low TMAO producers, high TMAO producers had some important microbiota differences, including lower alpha-diversity, different species compositions, and a higher ratio of Firmicutes to Bacteroidetes (about 2:1, versus a 1:1 ratio for the low producers). And very importantly, as we’ll see in a moment, the Archaea phylum was completely absent in the microbiota of the high TMAO producers, but was represented among the low TMAO producers.
This study provided some important pieces for the TMAO puzzle. Along with demonstrating that fish (at least in the form of cod) yields vastly higher levels of TMAO and TMAO metabolites than beef or eggs, the study demonstrated that TMAO can be absorbed intact from foods like fish without involvement of the gut microbiota. That means that the level of TMAO we have in our bodies isn’t just a result of our gut microbiota churning it out from precursors, but can also be influenced by the direct consumption of TMAO from food. And, that could mean that high circulating TMAO from seafood potentially indicates a much different (AKA lower!) risk profile than high TMAO produced by disease-associated microbes.
So, as much as is still up in the air about TMAO, one thing seems pretty likely: we don’t want a gut microbiome that produces a lot of it. Whether or not TMAO itself turns out to be a major risk factor or just a red herring, and whether or not our circulating levels are a cause or consequence of specific health conditions, a high-TMAO-generating microbiota seems to be consistent with greater disease risk.
Archaea: A Microbiome Missing Piece
Whenever we hear about the microbiota, it’s usually all about the bacteria, bacteria, and more bacteria! But, while bacteria might be numerically dominant among the microbes in our gut, they’re definitely not the only residents there. Another type of single-celled organism that call our GI tracts home are archaea. And, these little guys are incredibly important!
Archaea (sometimes called archaebacteria) are strict anaerobes that live in various mucosal sites throughout the body, including the intestinal mucosa. We aren’t born with archaea inside us, but we acquire them from the environment throughout life: by the time children are school-aged, archaea are almost universally present in the gut, and levels continue to increase with age (with the highest occurrence and diversity of archaea being found in older adults). Along with lacking lipopolysaccharide (meaning archaea won’t contribute to our endotoxin load), archaea is also the only domain of life that doesn’t include any known pathogens, which is pretty cool!
Archaea degrade TMA and TMAO
One important subtype of archaea are the methanogens, which produce methane gas as a byproduct of hydrogen reduction. Up to 95% of human guts harbor the methanogenic archaea Methanobrevibacter smithii and Methanosphaera stadtmanae, at varying levels. While the idea of producing methane might not sound very pleasant, some of these archaea (in particular, an order of methanogens called the Methanomassiliicoccales) actually play an integral role in reducing our TMAO production. Along with being able to use carbon dioxide, formate, and methanol (all released by bacteria breaking down food and other organic matter in the gut), these archaea can use methyl compounds like TMA and TMAO to generate methane. And that means that the archaea in our gut actually deplete the pool of TMA we have available to be converted into TMAO (along with any free TMAO from foods like seafood). So, all that TMA generated by Prevotella, or entering our body from fish consumption? Archaea can help wipe some of it out!
The TMAO-reducing effects of archaea is far from just speculative, too. In a study of ELDERMET subjects, the fecal TMA concentration in people with TMA-metabolizing archaea was significantly lower than in those without this archaea—and the difference was particularly dramatic when the abundance of Methanomassiliicoccales was greater than 10^8 cells per gram of stool. This role of archaea is so exciting that some scientists are proposing a new class of probiotics called archaeabiotics, which could help reduce our TMAO levels without us needing to cut back on choline and other important nutrients!
Importantly, methanogenic archaea have an important interactive relationship with bacteria in the gut, which often takes the form of what scientists call syntrophy (where two organisms participate in consuming a substance that neither one can catabolize on its own). And, it appears that archaea are particularly chummy with the important probiotic bacteria Bifidobacteria. For example, mixed cultures of M. smithii and Bifidobacteria bifidum have been shown to collectively produce methane from glucose, and in females with gut Methanobacteriales levels higher than 0.71%, there’s a significantly higher mean abundance of Bifidobacteriaceae. And as we’ve already seen, healthy levels of Bifidobacteria are super important!
Archaea Thrive When We Eat Carbs!
So, how do we keep our archaea happy and thriving? Scientists are just at the beginning stages of understanding how diet impacts our archaea colonies (after all, archaea are relative newcomers to the microbiota research scene compared to bacteria!), but here’s what we know so far. In children, organic dairy (especially organic yogurt and organic milk) has been associated with the initial colonization of M. smithii, due to organic dairy serving as a vehicle for delivering it to our guts. And, some methanogens degrade methanol, which gets produced when bacteria degrade pectin in fruits—thus suggesting that archaea might benefit from including fruit in our diets! In Chinese goats, eating a high-grain diet appears to suppress methanogenic archaea relative to a high-hay diet—possibly due to the pH-lowering effect of grains in the rumen (which can then suppress methanogenic archaea that are sensitive to low pH environments). Obviously, we’re not Chinese goats, but it would certainly be interesting if a high-grain diet in humans had a similar effect!
More broadly, though, in humans, Methanobrevibacter abundance is positively associated with higher carbohydrate consumption (both recent and long-term), and negatively associated with recent consumption of fat (especially vegetable fat and polyunsaturated fat intake) and amino acids. Although more research is definitely needed, the picture getting painted so far is that archaea benefit from a variety of plant polysaccharides, and not so much from animal-based diets.
However, this isn’t because the archaea themselves eat carbohydrate. In fact, methanogenic archaea have an almost complete lack of enzymes for breaking down complex carbohydrates into simple sugars. What does appear to be happening is that archaea thrive off the metabolic products of carbohydrate-loving bacteria, and therefore are still dependent on dietary carbohydrate for their own survival!
The Study: Long-term Paleolithic diet is associated with lower resistant starch intake, different gut microbiota composition and increased serum TMAO concentrations
 Whew! Now that we’ve covered the necessary background, let’s take a look at what this study is all about!
Whew! Now that we’ve covered the necessary background, let’s take a look at what this study is all about!
Researchers recruited a total of 44 participants who self-reported following a Paleo diet for over a year, along with 47 controls who more or less followed the national dietary recommendations of Australia (where the study was conducted). Within the Paleo group, participants were further divided based on how strictly they followed the Paleo framework (as gauged by the exclusion of grain and dairy products): 22 people fell into the “strict Paleo” group (less than one serving of grains and/or dairy per day), and 22 people fell into the “pseudo-Paleo” group (more than one serving of grains and/or dairy per day). (This is important, because “real-world” Paleo includes a variety of interpretations about what Paleo actually means, including how strictly to adhere to the diet—so a pseudo-Paleo group might be closer to reality for a lot of people!)
The study excluded anyone who’d taken antibiotics within the past six months, who had any type of GI tract surgery, who had past or present digestive disorders, who used cholesterol or blood pressure lowering medications, or who had been diagnosed with cardiovascular disease. This helped make sure the results weren’t confounded by preexisting health conditions or drugs, and allowed the researchers to better isolate the effects of the participants’ diets.
To ensure accurate dietary data, the researchers had participants undergo three-day weighed diet records (including two weekdays and one weekend day), which were then validated using urine nitrogen tests (which could detect whether participants were over- or under-reporting their protein intake) and the Goldberg cut point (which can identify which participants were under-reporting their energy intake, through the ratio of energy intake to basal metabolic rate). Participants who appeared to inaccurately report both their protein and energy intake were deemed unreliable dietary reporters (this ended up being two people from the strict Paleo group and three people from the control group).
Paleo Diet Increases TMAO
Now, the headline-grabbing finding. In the strict Paleo group, serum TMAO was a whopping 9.53 µM, the pseudo-Paleo group averaged 5.47 µM, and the control group averaged 3.93 µM. Higher TMAO levels were associated positively with red meat intake and negatively with grain intake.
Whether TMAO is a causative agent for disease or a marker for a disease-associated microbiota, those are some scary numbers—especially for the strict Paleo group!
What Study Participants Were Actually Eating
So, what were the Paleo groups actually eating? Here’s what the data showed!
 Not surprisingly, both Paleo groups ate more protein than the controls (118 and 102.7 grams per day for the strict Paleo group and pseudo-Paleo group, respectively, compared to 92 grams for the controls), less carbohydrate (99 and 81.4 grams per day for the strict Paleo group and pseudo-Paleo group, respectively, compared to 202.6 grams for the controls), less sugar (51.8 and 44 grams per day for the Paleo groups, and 75.6 grams for the controls), and more fat—with the increase mostly coming from saturated and monounsaturated forms (117.9 total fat grams per day for the strict Paleo group, 133.1 grams for the pseudo-Paleo group, and 82.5 grams for the controls).
Not surprisingly, both Paleo groups ate more protein than the controls (118 and 102.7 grams per day for the strict Paleo group and pseudo-Paleo group, respectively, compared to 92 grams for the controls), less carbohydrate (99 and 81.4 grams per day for the strict Paleo group and pseudo-Paleo group, respectively, compared to 202.6 grams for the controls), less sugar (51.8 and 44 grams per day for the Paleo groups, and 75.6 grams for the controls), and more fat—with the increase mostly coming from saturated and monounsaturated forms (117.9 total fat grams per day for the strict Paleo group, 133.1 grams for the pseudo-Paleo group, and 82.5 grams for the controls).
Importantly, total dietary fiber was adequate (and very similar!) for both the control group and the strict Paleo group—29.7 grams and 27.4 grams daily, respectively, which falls within the recommended daily intake of 25-30 grams for adults. The pseudo-Paleo group averaged 20.8 grams of fiber each day, which is still more than most people consume! For the Paleo groups, much of their fiber came from vegetables, with the strict Paleo group eating 6.7 servings of veggies per day and the pseudo-Paleo group averaging 4.3, compared to the control group’s 3.93. (We could definitely argue that the official recommendations for fiber are still too low for optimal health, but the point here is, these study participants weren’t slacking on the non-starchy veggies!)
While the control group averaged 4.5 – 14.2 grams of resistant starch per day, largely from grains (those are estimated minimum and maximum amounts, since the same foods can vary in resistant starch content), the strict Paleo group averaged only 2.6 – 6.1 grams per day, and the pseudo-Paleo group got a mere 1.3 – 2.9 grams of resistant starch. That’s a significant drop off!
Low-Carb Paleo Effects on the Microbiome
Remember that one of the favorite foods of Bifidobacteria is resistant starch? And that Roseburia also likes to munch on carbohydrates like beta-glucans? Maybe we shouldn’t be surprised that this study showed a significant decrease in these important bacteria among both Paleo groups.
Likewise, the relative abundance of Hungatella was significantly higher among the Paleo subjects. After adjusting for age, gender, stool frequency, and body fat, Hungatella abundance was significantly negatively associated with grain intake among the study’s participants, and was also significantly negatively correlated with Bifidobacteria and Roseburia abundance. Although we don’t have a lot of information about Hungatella in relation to human health (not yet, anyway!), we do know that some species originating from the genus Hungatella are associated with choline consumption and TMA production, including at least one species identified in this study. The researchers speculated that some components of grains and/or whole grains either interfere with Hungatella’s TMA production or prevent it from dominating in the gut. And, these components might not be resistant starch, since neither Hungatella nor TMAO was significantly associated with resistant starch intake!
Resistant Starch and Total Carb Intake Are Important!
One of the things that makes this study so interesting (and important!) is the fact that fiber and vegetable intake was relatively high for both Paleo groups. Often, when we talk about how ketogenic diets or low-carb or low-starch Paleo might affect the gut microbiome, we’re given a false sense of security thinking we’ll be fine as long as we eat plenty of fiber from leafy greens; see also How Ketogenic Diet Wreaks Havoc on Your Gut. As this study indicates, low-starch veggies alone are not enough to maintain a robust, diverse gut microbiome! The Paleo subjects clearly weren’t ingesting a broad enough array of fibers and starches to support some very important bacterial populations.
Given all that, can we just add some supplemental resistant starch to otherwise low-carb diet and call it a day?
Actually, the answer here seems to be no! Consistent with my post Resistant Starch: It’s Not All Sunshine and Roses, the research shows that isolated resistant starch doesn’t bring the same range of benefits as resistant starch from whole-food sources. And what’s more, when it comes to TMAO, supplemental resistant starch may even be counterproductive in the context of a low-carb and/or high fat diet. In a human cross-over trial from 2016, for example, 52 adults consumed four different diets in random orders—a low resistant starch, higher carbohydrate diet; a low resistant starch, lower carbohydrate diet; a high resistant starch, higher carbohydrate diet; and a high resistant starch, lower carbohydrate diet (with resistant starch coming from Hi-Maize 260, supplying RS2). Intriguingly, the highest TMAO levels occurred when subjects were eating the high resistant starch, lower carbohydrate diet, despite dietary levels of carnitine and choline being less on that diet compared to the low resistant starch periods. The researchers proposed that a high isolated resistant starch intake, in the presence of an overall lower carbohydrate diet, shifted the microbiota towards greater TMAO generation. Likewise, studies in rodents have shown that high fat consumption (which often goes hand-in-hand with low-carbohydrate diets), in the amount of of 42% of total energy, partially counteracts the beneficial effects of RS2 by suppressing levels of beneficial bacteria. So, low-carb diets can be a double whammy against our gut microbiota, both by failing to supply a wide range of fiber and starch types and by overloading our guts with levels of fat that become harmful to bacteria. (Don’t worry; we aren’t calling fat bad here—but a balanced intake of macronutrients seems to be where the evidence is pointing for optimal health!)
Other studies support the idea that resistant starch is most health-promoting in conjunction with other dietary carbohydrates. In pigs, resistant starch alone (in the form of RS2) was shown to get rapidly fermented in the proximal (beginning part) of the colon, while failing to reach further down into the distal (lower) colon—resulting in only a small portion of the colon receiving fermentation benefits. But, when additional carbohydrate in the form of wheat bran (a soluble non-starch polysaccharide) was included in the pigs’ diets, the amount of resistant starch getting fermented between the lower colon and feces nearly doubled—indicating that the bulk from the wheat bran was helping spread fermentation further down through the colon, flooding a much greater area with cancer-protective butyrate. The addition of wheat seed (RS1) to supplemental green banana flour and high-amylose starch (RS2) has been shown to help spread fermentation throughout the entire colon, as indicated by a decrease in fecal pH (which is a good thing!).
When it comes to low-carb Paleo diets, it’s easy to see how a similar effect could occur. Cutting out sources of bulky fermentable carbohydrate (like root veggies and legumes) limits how far isolated resistant starch can spread, resulting in bacteria in the proximal colon gobbling it up and leaving none for microbes further down the colon. Likewise, given what we know about the role of methanogenic archaea in regulating TMAO levels and interacting with other microbes like Bifidobacteria (as well as how much methanogenic archaea likes carbohydrates and doesn’t like fat!), we might suspect that low-carb diets can also enhance TMAO production by suppressing archaea growth.
As further confirmation, the main study we’ve been discussing in this article found that TMAO levels were more strongly negatively associated with grain intake than with resistant starch intake. That doesn’t mean we all need to eat grains to be healthy, but it does imply that diverse components of starchy plant foods (such as the other forms of fiber and carbohydrate they contain) contribute to a healthy microbiome, even more so than resistant starch on its own.
Short-Term Vs. Long-Term Microbiome Shifts
We should also stress the importance of the long-term nature of this study! While shorter dietary trials can be very useful (and in some cases, are the only studies we have at our disposal), they can’t capture potential health issues that develop months or years down the line (such as gradual changes in the core gut microbiota composition). That’s particularly relevant here, because the same researchers who conducted this study previously published a similar one, randomizing 22 women to a Paleo diet for four weeks and 17 women to a diet in line with Australian health recommendations. In that study, there wasn’t a significant change in TMAO concentration compared to the control group, despite a lower intake of resistant starch and higher intake of meat and eggs among the Paleo dieters. While those findings could have been due to the small sample size and limited data for resistant starch content of foods (making it difficult to precisely calculate resistant starch intake), it’s also possible that the Paleo group hadn’t yet exhibited some longer-term microbiota shifts that lead to higher TMAO generation as seen in the more recent study.
Indeed, the field of microbiota research has shown us that while some microbial changes happen rapidly when we alter our diet, others can occur on a more gradual basis. So, only using participants who had been eating Paleo for over a year gives this study greater insight into the full effects that low-carb Paleo has on the gut microbiota.
The Bottom Line: Paleo Should Not Be Low-Carb
Some people have seen this paper as an attack on red meat, which, given the TMAO link, is a valid concern (see also The Link Between Meat and Cancer). But what this paper is actually making a case for is that starch-free diets are a problem. Don’t get me wrong: fibrous, low-starch veggies are fantastic, and we should be filling our plates with them on the daily—but not at the exclusion of Paleo starches! Root veggies rich in resistant starch are consistently showing up as a vital component of a nutrient-dense Paleo diet, and there are no real “hacks” to get around that (see also Why Root Veggies Are Great for the Gut Microbiome and Resistant Starch: It’s Not All Sunshine and Roses).
When we combine this information with an examination of the non-metabolic roles that insulin plays (another rationale for moderate [not low] carb intake, see The Case for More Carbs: Insulin’s NonMetabolic Roles in the Human Body and How Many Carbs Should We Eat?), the potential problems of too-high-fat intake (not that we wan’t to eat low-fat; see Saturated Fat: Healthful, Harmful, or Somewhere In Between?, Adverse Reactions to Ketogenic Diets: Caution Advised), and understanding that micronutrient sufficiency is most easily attained with a balanced macronutrient diet (see The Diet We’re Meant to Eat, Part 3: How Much Meat versus Veggies?, The Importance of Nutrient Density, and Carbs Vs. Protein Vs. Fat: Insight from Hunter-Gatherers), it’s becoming harder and harder to make a compelling case against moderate carbohydrate intake, 30 to 60% of total calories, from whole food sources.
Citations
Bergeron N, et al. “Diets high in resistant starch increase plasma levels of trimethylamine-N-oxide, a gut microbiome metabolite associated with CVD risk.” Br J Nutr. 2016 Dec;116(12):2020-2029. doi: 10.1017/S0007114516004165. Epub 2016 Dec 20.
Borrel G, et al. “Genomics and metagenomics of trimethylamine-utilizing Archaea in the human gut microbiome.” ISME J. 2017 Sep;11(9):2059-2074. doi: 10.1038/ismej.2017.72. Epub 2017 Jun 6.
Charrier JA, et al. “High fat diet partially attenuates fermentation responses in rats fed resistant starch from high-amylose maize.” Obesity (Silver Spring). 2013 Nov;21(11):2350-5. doi: 10.1002/oby.20362. Epub 2013 Jul 2.
Cho, CE, et al. “Trimethylamine‐N‐oxide (TMAO) response to animal source foods varies among healthy young men and is influenced by their gut microbiota composition: A randomized controlled trial” M. A., Mol. Nutr. Food Res. 2016, 1600324. Doi: 10.1002/mnfr.201600324
Dridi B, et al. “High prevalence of Methanobrevibacter smithii and Methanosphaera stadtmanae detected in the human gut using an improved DNA detection protocol.” PLoS One. 2009 Sep 17;4(9):e7063. doi: 10.1371/journal.pone.0007063.
Dridi B, et al. “High prevalence of Methanobrevibacter smithii and Methanosphaera stadtmanae detected in the human gut using an improved DNA detection protocol.” PLoS One. 2009 Sep 17;4(9):e7063. doi: 10.1371/journal.pone.0007063.
Genoni, A, et al. “Long-term Paleolithic diet is associated with lower resistant starch intake, different gut microbiota composition and increased serum TMAO concentrations.” European Journal of Nutrition, 2019; DOI: 10.1007/s00394-019-02036-y
Genoni A, et al. “A Paleolithic diet lowers resistant starch intake but does not affect serum trimethylamine-N-oxide concentrations in healthy women.” Br J Nutr. 2019 Feb;121(3):322-329. doi: 10.1017/S000711451800329X. Epub 2018 Nov 13.
Govers MJ, et al. “Wheat bran affects the site of fermentation of resistant starch and luminal indexes related to colon cancer risk: a study in pigs.” Gut. 1999 Dec;45(6):840-7.
Hoffmann C, et al. “Archaea and fungi of the human gut microbiome: correlations with diet and bacterial residents.” PLoS One. 2013 Jun 17;8(6):e66019. doi: 10.1371/journal.pone.0066019.
Jin W, Cheng Y, Zhu W. “The community structure of Methanomassiliicoccales in the rumen of Chinese goats and its response to a high-grain diet.” J Anim Sci Biotechnol. 2017 Jun 1;8:47. doi: 10.1186/s40104-017-0178-0.
Qi J, et al. “Circulating trimethylamine N-oxide and the risk of cardiovascular diseases: a systematic review and meta-analysis of 11 prospective cohort studies.” J Cell Mol Med. 2018 Jan;22(1):185-194. doi: 10.1111/jcmm.13307. Epub 2017 Aug 7.
Raymann K, et al. “Unexplored Archaeal Diversity in the Great Ape Gut Microbiome.” mSphere. 2017 Feb 22;2(1). pii: e00026-17. doi: 10.1128/mSphere.00026-17.
Tamanai-Shacoori Z, et al. “Roseburia spp.: a marker of health?” Future Microbiol. 2017 Feb;12:157-170. doi: 10.2217/fmb-2016-0130.
van de Pol JA, et al. “Gut Colonization by Methanogenic Archaea Is Associated with Organic Dairy Consumption in Children.” Front Microbiol. 2017 Mar 10;8:355. doi: 10.3389/fmicb.2017.00355. eCollection 2017.
Vanderhaeghen S, Lacroix C, Schwab C. “Methanogen communities in stools of humans of different age and health status and co-occurrence with bacteria.” FEMS Microbiol Lett. 2015 Jul;362(13):fnv092. doi: 10.1093/femsle/fnv092.
Vanessa DN, et al. “Archaea: Essential inhabitants of the human digestive microbiota.” Human Microbiome Journal, 2017 3:1-8. doi.org: 10.1016/j.humic.2016.11.005.












