When we consume carbohydrates, our blood sugar increases. In response to that rise in blood sugar, the pancreas releases the hormone insulin, which facilitates the transport of glucose into the cells of the body (via the glucose transporter GLUT4) and signals to the liver to convert glucose into glycogen for short-term energy storage in liver and muscle tissues and into triglycerides for long-term energy storage in adipose tissues.
Table of Contents[Hide][Show]
It’s a beautifully efficient system… until things go wrong. Chronically elevated blood sugar levels stimulate adaptations within cells, rendering them less sensitive to insulin. These adaptations may include decreasing the number of receptors to insulin embedded within the cell membranes and suppressing the signaling within the cell that occurs after insulin binds to its receptor. This causes the pancreas to secrete more insulin to lower the elevated blood glucose levels. This is called insulin resistance or loss of insulin sensitivity, when more insulin than normal is required to deal with blood glucose. When normal blood sugar levels can no longer be maintained, you get type 2 diabetes. (In contrast, type 1 diabetes mellitus is an autoimmune disease where the beta cells of the pancreas are attacked and destroyed, so the pancreas’ ability to produce and release insulin is diminished; see The Autoimmune Protocol. This is why insulin injections or an insulin pump are the typical treatment for type 1 diabetics but diabetes treatment for type 2 diabetics more often includes diet and lifestyle changes as well as prescribed oral medications like metformin.)
Both hyperglycemia and hyperinsulinemia (high blood sugar and insulin, respectively) are independently inflammatory. So, even if normal blood sugar levels can be maintained, if the pancreas is secreting more insulin than normal, both basal levels of inflammation increase and the response to an inflammatory stimulus is exaggerated.
Insulin resistance is bad. It promotes weight gain and increases risk not only of obesity and diabetes, but also cardiovascular disease, many forms of cancer, asthma, allergies, PCOS, chronic kidney disease, many autoimmune diseases…. the list goes on. (See also The Hormones of Hunger, The Hormones of Fat: Leptin and Insulin and How many carbs should you eat?)
So, if chronically elevated blood sugar levels makes you insulin resistant leading to health problems, then the key must be not to eat all those carbs, right? This thinking is what led to the now-debunked carbohydrate-insulin hypothesis of obesity and a surge in popularity of low-carb diets since the early 1990s.
Low-carb diets do promote weight loss (and achieving a healthy weight reduces risk of diabetes, cardiovascular disease, etc.). This fact has led to their increased popularity and the push towards ever lower carbohydrate intake, including the current ketogenic diet fad. However, rigorous and well-controlled metabolic ward studies have confirmed that low-carb and ketogenic diets don’t turn us into “fat-burning machines” with increased energy expenditure and preferential fat mass loss. The truth might actually be the opposite. These diets help us lose weight simply by creating a dietary structure that focuses on more satiating foods so that most individuals naturally achieve a caloric deficit while following these dietary templates. While weight loss is certainly more complicated than calories in versus calories out, a caloric deficit is still a necessary condition for weight loss. When followed for long periods, ketogenic diets can tank our metabolism and have a muscle-wasting effect, making weight loss maintenance particularly challenging. See also New Scientific Study: Calories Matter, Adverse Reactions to Ketogenic Diets: Caution Advised, How Ketogenic Diet Wreaks Havoc on Your Gut , Paleo for Weight Loss and Can You Really Be Healthy at Any Size?.
The fact is that human insulin plays a lot of other important roles in human health independent of its role in energy balance. So, while insulin resistance is clearly damaging, not eating enough carbs to support healthy amounts of insulin secretion can also lead to similar health problems and make long-term weight loss maintenance tougher.
So, in examining the case for more carbs (healthy whole-foods ones, of course), let’s start with a deep dive into the physiologic roles of insulin beyond glucose metabolism
The Diverse, Non-Glucose-Related Roles of Insulin
Insulin is a superhormone, with an array of functions in human physiology that far exceed its simplistic characterization as the metabolic hormone responsible for shuttling glucose into our cells to be converted into ATP (adenosine triphosphate, the energy currency of all cells). (Another example of a superhormone is cortisol, see How Stress Undermines Health and Regulating Circadian Rhythm (and why that’s important)).
In fact, insulin does several very important jobs that have nothing to do with glucose. And once we discover and recognize these other roles of insulin, it’s easy to understand why too little insulin (or hindered insulin signaling as occurs in insulin resistance) may have some negative consequences.
Insulin and Thyroid Function
 The thyroid gland produces hormones that control metabolism as well as influence other essential systems in the human body, such as the cardiovascular system, the immune system, and calcium homeostasis. Thyroid hormones increase our basal metabolic rate, control appetite, improve absorption of nutrients from the digestive tract, and control gut motility. They play an essential role in glucose metabolism and also stimulate the breakdown of fats.
The thyroid gland produces hormones that control metabolism as well as influence other essential systems in the human body, such as the cardiovascular system, the immune system, and calcium homeostasis. Thyroid hormones increase our basal metabolic rate, control appetite, improve absorption of nutrients from the digestive tract, and control gut motility. They play an essential role in glucose metabolism and also stimulate the breakdown of fats.
Nutrivore Weekly Serving Matrix
An easy-to-use and flexible weekly checklist
to help you maximize nutrient-density.
The Weekly Serving Matrix is very helpful! I’ve been eating along these lines but this really helps me know where to focus vs. which foods serve a more secondary role. It’s super helpful and has taken a lot of worry out of my meal planning. Thanks!
Jan
The prohormone thyroxine (T4) is produced in the thyroid gland and is then converted into the more active thyroid hormone triiodothyronine (T3) by a group of selenium-containing enzymes called deiodinases (aka 5′-iodinase) that are found in multiple areas of the body. Type 1 deiodinase (D1) is present in the thyroid, liver and kidney. Type 2 deiodinase (D2) is present in the thyroid, central nervous system, pituitary (making its activity the most important for negative feedback on thyroid-stimulating hormone), pineal gland, brown adipose tissue (important in cold adaptation), placenta, skeletal muscle, and heart. Type 3 deiodinase (D3) is found in the placenta and central nervous system.
Type 2 deiodinase is approximately 1000 times more active than either type 1 or type 3 and D2-catalyzed T3 production increases thyroid hormone signaling (blocking D2 causes localized hypothyroidism in various tissues). Importantly, its activity is upregulated by insulin and is decreased during fasting. It is well known that insulin (in the absence of insulin resistance) can stimulate the conversion of T4 to T3 via its effect on D2 activity.
Insulin and Skeletal Muscle Metabolism
 Along with aesthetics and athletic performance, building muscle protects against insulin resistance and diabetes and having higher muscle mass is associated with reduced risk of cardiovascular disease. A variety of studies demonstrate that muscle mass is a better predictor of all-cause mortality than BMI, meaning, it’s more important to build muscle than to lose fat from a health and longevity standpoint.
Along with aesthetics and athletic performance, building muscle protects against insulin resistance and diabetes and having higher muscle mass is associated with reduced risk of cardiovascular disease. A variety of studies demonstrate that muscle mass is a better predictor of all-cause mortality than BMI, meaning, it’s more important to build muscle than to lose fat from a health and longevity standpoint.
Beyond the obvious roles that insulin has in stimulating uptake of glucose and triglycerides from the blood into muscle cells to use as energy, insulin is also critical for protein metabolism in muscle independent of energy status. In particular, insulin exerts anabolic and anti-catabolic effects (meaning insulin is important for muscle growth and repair).
Insulin increases the rate of transport of some amino acids into muscle tissue—including the branched-chain amino acids leucine, isoleucine and valine, plus arginine, phenylalanine and lysine—making them available for muscle protein synthesis. Insulin stimulates muscle protein synthesis in the presence of elevated amino acids, such as occurs after exercise thanks to increased blood flow in muscles. Insulin also reduces muscle protein breakdown independent of amino acid availability (and it might reduce protein degradation in other tissues too). Interestingly, these anabolic effects can even be observed in the case of insulin resistance if supraphysiologic doses of insulin are administered(as in the case of insulin treatment or insulin therapy).
Insulin and Bone Health
 Even after we’re fully grown, our bones are constantly being remodeled—bone tissue is both resorbed (broken down), which is driven by a cell type called osteoclasts, and formed, which is driven by a cell type called osteoblasts. Osteoclasts erode the surface of bone by secreting enzymes that degrade the protein matrix and acids that solubilize bone minerals. Osteoblasts form new bone by secreting the protein matrix and then gradually mineralizing the matrix by secreting hydroxyapatite crystals.
Even after we’re fully grown, our bones are constantly being remodeled—bone tissue is both resorbed (broken down), which is driven by a cell type called osteoclasts, and formed, which is driven by a cell type called osteoblasts. Osteoclasts erode the surface of bone by secreting enzymes that degrade the protein matrix and acids that solubilize bone minerals. Osteoblasts form new bone by secreting the protein matrix and then gradually mineralizing the matrix by secreting hydroxyapatite crystals.
Insulin modulates bone growth and turnover by increasing osteoblast activity, proliferation, differentiation and survival, and promoting collagen synthesis. Insulin resistance is associated with reduced bone mass and increased fracture rates. This link goes the other way too though. In response to insulin, osteoblasts increase secretion of the hormone osteocalcin, which increases the efficiency of glucose metabolism in the rest of the body. Under normal conditions, insulin-responsive bone cells help regulate global energy homeostasis. When insulin signaling is impaired, bone cells contribute to the vicious cycle of glucose intolerance and insulin resistance.
Insulin and the Central Nervous System
 Circulating insulin crosses the blood-brain barrier where it binds with neuron and astrocyte insulin receptors in various areas of the brain. Insulin signaling in the hypothalamus as a hunger hormone and adiposity signal is well understood and relates to glucose homeostasis and energy balance. However insulin also acts in the hippocampus independent of its glucoregulatory effects, impacting cognitive functions including promoting learning and memory. There’s also evidence that insulin is important for synaptic plasticity because it modulates the activity of important neurotransmitter receptors, such as those for glutamate and GABA.
Circulating insulin crosses the blood-brain barrier where it binds with neuron and astrocyte insulin receptors in various areas of the brain. Insulin signaling in the hypothalamus as a hunger hormone and adiposity signal is well understood and relates to glucose homeostasis and energy balance. However insulin also acts in the hippocampus independent of its glucoregulatory effects, impacting cognitive functions including promoting learning and memory. There’s also evidence that insulin is important for synaptic plasticity because it modulates the activity of important neurotransmitter receptors, such as those for glutamate and GABA.
Defective insulin signaling in the brain (such as in insulin resistance) is associated cognitive impairment and may be the mechanism behind symptoms of dementia in people with age-related neurodegenerative diseases like Alzheimer’s disease as well as the neurological complications of diabetes.
Insulin also impacts neuroinflammation by binding to receptors on microglia cells, the resident macrophages (sentinel immune cells) of the brain. Pathological microglial activation contributes to the development and progression of neurodegenerative disorders via neurotoxicity of secreted pro-inflammatory cytokines (chemical messengers of inflammation). Studies have shown that when insulin concentrations are low or signaling is inhibited (as occurs in Alzheimer’s disease), microglia cell activity increases and they secrete more pro-inflammatory cytokines. At higher insulin concentrations, neurotoxicity of stimulated microglia cells is reduced. In fact insulin (and insulin-like growth factor) have become potential therapeutic targets and studies in models of traumatic brain injury and Alzheimer’s disease have shown improved cognition and reduced neuroinflammation with intranasal application of insulin.
 Insulin and Hormone Regulation
Insulin and Hormone Regulation
Insulin influences the levels of a variety of hormones, often interacting with hormone systems in complex and reciprocal ways. This can contribute to a snowball effect once insulin resistance begins.
For example, insulin acts synergistically with insulin-like growth factor 1 and follicular stimulating hormone to enhance estrogen and testosterone production. Insulin also impacts levels of sex-hormone binding globulin (SHBG), which is a circulating hormone which binds to testosterone and estrogen in the blood, rendering them inactive. SHBG is important for regulating the balance between testosterone and estrogen, as well as helping to transport the sex hormones into tissues. High levels of insulin are associated with low levels of SHBG, so not only are more sex hormones being produced, but they’re also more active because they’re not bound to SHBG. In women, hyperinsulinemia may lower estrogen levels but low estrogen levels increase insulin resistance. Because the system is complex, insulin resistance is also associated with estrogen excess in women in some circumstances.
Similar antagonistic effects of insulin are observed with other hormones. Due to insulin’s antagonistic effect on cortisol, insulin resistance causes increased cortisol but high cortisol increases insulin resistance. Insulin also interacts with growth hormone, glucagon, and hormone neurotransmitters like dopamine, serotonin and melatonin. These interactions are incredibly multifaceted, but what can be said for certain is that maintaining insulin sensitivity is necessary for balance among diverse hormone systems.
Insulin and Immune Health
 Insulin signaling affects the full range of immune cell populations and functions beyond glucose transport and glycolysis.
Insulin signaling affects the full range of immune cell populations and functions beyond glucose transport and glycolysis.
In the innate immune system, insulin signaling activates neutrophils (the first line of defense cells during infection or injury), regulates their migration from the blood in to tissues, and stimulates phagocytosis (the process of engulfing pathogens). Similarly, insulin signaling increases that phagocytic activity of macrophages (sentinel immune cells resident in all tissues in the body) and activates dendritic cells (sentinel immune cells found in barrier tissues). Insulin also enhances the cytotoxicity of natural killer cells (whose job it is to destroy virally-infected cells and cancerous cells). When insulin levels are elevated, these effects result in generalized inflammation; however, when insulin resistance becomes advanced, the activity of these innate immune system cells becomes impaired.
In the adaptive immune system, insulin activates CD4+ T cells (which include the full range of helper T cells, also known as effector cells since they drive immune attacks, as well as regulatory T cells, which modulate immune responses and prevent autoimmune disease) and increases the cytotoxicity of CD8+ T cells (which includes cytotoxic T cells, who function similarly to natural killer cells). When insulin is high, Th1 and Th17 helper T cell subsets (whose overactivity are implicated in allergic, immune and autoimmune conditions) become more stable and resistant to modulation by external factors, and Tr1 cells (an induced regulatory helper T cell subtype) disappear. Blocking insulin signaling results in lower cytokine production, reduced proliferation, reduced migration, and increased apoptosis of CD4+ T cells including reduced activity of regulatory T cells.
These specific impacts of insulin signaling on immune cells implies that insulin is required for a normal and healthy immune response. It also explains why high insulin or insulin resistance is inflammatory, but also why insulin resistance is associated with allergic, inflammatory and autoimmune diseases and suppression of the immune system’s ability to fight off infection.
The Forgotten C-Peptide
The roles of insulin in the human body are impressive, but let’s not forget that insulin is never released on its own.
Proinsulin is produced in the pancreas and when blood sugar levels increase, proinsulin is rapidly cleaved in two, forming the active hormone insulin and the oft forgotten C-peptide. Scientists used to think that C-peptide was little more than a clever switch (remove it from insulin to turn it on, making for a rapid acting system) and an efficient way to be able to release a lot of insulin quickly upon demand.
But, we now recognize that C-peptide is biologically active, with potent antioxidant and anti-inflammatory properties. For example, C-peptide binds to receptors in cell membranes and triggers an intracellular signaling cascade that results in upregulation of endogenous antioxidant enzymes (like the very beneficial endogenous Nitric Oxide Synthase, aka eNOS).
 C-peptide appears to be an important protector against many of the deleterious effects of chronically elevated blood glucose, including protecting the brain, kidneys, retinas and vasculature (neuropathy, renal failure, retinopathy and peripheral vascular diseases are common complications of diabetes). In fact, an array of recent studies are showing therapeutic benefit of C-peptide administration in diabetics. Levels of C-peptide in diabetics is also a good predictor of risk for complications of diabetes and poorer metabolic control.
C-peptide appears to be an important protector against many of the deleterious effects of chronically elevated blood glucose, including protecting the brain, kidneys, retinas and vasculature (neuropathy, renal failure, retinopathy and peripheral vascular diseases are common complications of diabetes). In fact, an array of recent studies are showing therapeutic benefit of C-peptide administration in diabetics. Levels of C-peptide in diabetics is also a good predictor of risk for complications of diabetes and poorer metabolic control.
It’s likely that many of the benefits of moderate intake of whole-food carbohydrates (maintaining insulin sensitivity) is attributable to the activities of C-peptide.
Too Little Insulin versus Insulin Resistance
While the utility of ketogenic diets in refractory epilepsy, neurodegenerative disorders and GLUT1 deficiency syndrome is undisputed, its general use may do more harm than good. The evidence is mounting that very low-carbohydrate diets, such as ketogenic diets, have negative effects on our health that are often analogous to insulin resistance. In diabetes, the body is less sensitive to insulin signaling. During very low-carb intake, insufficient insulin is secreted for normal signaling.
Studies have identified similar detriments to thyroid function, skeletal muscle health, bone density, mental health and cognition, and immune function between type 2 diabetes and very low-carbohydrate ketogenic diets. These similarities in adverse effects are likely attributable to the roles that insulin plays in human health beyond glucose homeostasis.
Hypothyroidism
 Ketogenic diets are known to decrease thyroid function. In fact, in one recent study following 120 pediatric epilepsy patients following a Mediterranean-style ketogenic diet for seizure control showed that a whopping 1 in 6 (20 out of the 120) study participants developed hypothyroidism (with a significant drop in free T3 and concurrent rise in TSH) requiring L-thyroxine medication within the first 6 months of the study! And 8 of the 20 participants who developed hypothyroidism did so within the first month! Interestingly, females were more likely to develop hypothyroidism as a result of following a ketogenic diet. Thyroid suppression was evidence in all study participants, with an overall significant increase in TSH and decrease in free T3. In a variety of other human and animal studies, reduced body temperature, fatigue, lethargy and torpor are also reported as side effects of a ketogenic diet (likely due to reduced thyroid hormone levels). Of course, weight loss in general can reduce T3 and many defenders of ketogenic diets for weight loss cling to this fact. However, the decrease in free T3 during weight loss is in large part because T3 tends to be elevated in obesity and weight loss normalizes it. Studies that have investigated the effect of weight loss via calorie restriction on thyroid function have not identified an increased risk of hypothyroidism (meaning that even though T3 decreases, it remains within normal lab ranges).
Ketogenic diets are known to decrease thyroid function. In fact, in one recent study following 120 pediatric epilepsy patients following a Mediterranean-style ketogenic diet for seizure control showed that a whopping 1 in 6 (20 out of the 120) study participants developed hypothyroidism (with a significant drop in free T3 and concurrent rise in TSH) requiring L-thyroxine medication within the first 6 months of the study! And 8 of the 20 participants who developed hypothyroidism did so within the first month! Interestingly, females were more likely to develop hypothyroidism as a result of following a ketogenic diet. Thyroid suppression was evidence in all study participants, with an overall significant increase in TSH and decrease in free T3. In a variety of other human and animal studies, reduced body temperature, fatigue, lethargy and torpor are also reported as side effects of a ketogenic diet (likely due to reduced thyroid hormone levels). Of course, weight loss in general can reduce T3 and many defenders of ketogenic diets for weight loss cling to this fact. However, the decrease in free T3 during weight loss is in large part because T3 tends to be elevated in obesity and weight loss normalizes it. Studies that have investigated the effect of weight loss via calorie restriction on thyroid function have not identified an increased risk of hypothyroidism (meaning that even though T3 decreases, it remains within normal lab ranges).
Insulin resistance, obesity and diabetes are also linked with altered thyroid function, but both hypothyroidism and hyperthyroidism are possible consequences (the mechanistic distinctions between these two outcomes are still being investigated). The prevalence of thyroid disease, including subclinical hypothyroidism (low thyroid function but not low enough for medication), in patients with obesity or diabetes is significantly higher than that in the general population. This is attributable to effects of insulin on T4 to T3 conversion but also the effects of thyroid hormones on insulin sensitivity (via changes in LDL cholesterol)!
Reduced Performance and Loss of Lean Mass
 People with type 2 diabetes experience poor muscle strength and function, and the rate at which muscle quality and strength decline with age is approximately 30% faster than in healthy individuals. On the flip side of this coin, a number of human and animal studies have identified loss of lean mass (skeletal muscle) during weight loss using a ketogenic diet. While all rapid weight loss diets result in loss of lean mass in addition to fat mass, when a hypocaloric ketogenic diet was pitted against a nonketogenic diet matched for calories and protein intake, there was greater protein sparing by the nonketogenic while total weight loss was equivalent between the two groups. The authors identify greater protein synthesis in the nonketogenic diet group as the mechanism for the protein sparing effect (which makes sense knowing that insulin stimulates muscle protein synthesis). Maintaining lean mass through weight loss is highly desirable and the body of evidence supports higher protein intake combined with physical activity is the best ways to achieve this goal.
People with type 2 diabetes experience poor muscle strength and function, and the rate at which muscle quality and strength decline with age is approximately 30% faster than in healthy individuals. On the flip side of this coin, a number of human and animal studies have identified loss of lean mass (skeletal muscle) during weight loss using a ketogenic diet. While all rapid weight loss diets result in loss of lean mass in addition to fat mass, when a hypocaloric ketogenic diet was pitted against a nonketogenic diet matched for calories and protein intake, there was greater protein sparing by the nonketogenic while total weight loss was equivalent between the two groups. The authors identify greater protein synthesis in the nonketogenic diet group as the mechanism for the protein sparing effect (which makes sense knowing that insulin stimulates muscle protein synthesis). Maintaining lean mass through weight loss is highly desirable and the body of evidence supports higher protein intake combined with physical activity is the best ways to achieve this goal.
Studies of ketogenic diets in elite endurance athletes demonstrate impaired performance (despite some benefits to aerobic capacity) because the higher rate of fat oxidation during ketosis causes increased oxygen demand. Studies have also identified reduced anaerobic exercise performance following ketogenic diets, which applies to high-intensity, short duration activities and sports. There are some studies using amino acid supplements in ketogenic diets attempting to mitigate these unwanted effects.
Reduced Bone Mineral Density
 Individuals with type 2 diabetes mellitus (T2DM) have a 69% increased risk of bone fractures and, because bone remodeling is around 70% lower in diabetics than healthy people, healing of bone fractures can take substantially longer than normal.
Individuals with type 2 diabetes mellitus (T2DM) have a 69% increased risk of bone fractures and, because bone remodeling is around 70% lower in diabetics than healthy people, healing of bone fractures can take substantially longer than normal.
A recent study of 29 pediatric epilepsy patients following a ketogenic diet for an average of 2.1 years showed significant loss of bone mineral density (both whole body and lumbar spine) with two children sustaining fractures during the study period. These results confirmed an earlier study in which loss of bone mineral density was even higher, attributable at least in part to the fact that half of the children included in that study started with lower-than-average bone mineral content. Only one study in adults has measured bone mineral density and no significant decline was observed over 5 years. However, effects of ketogenic diets on bone health is more readily observed in pediatric populations since children have higher rates of bone turnover and growth.
Impaired Memory, Mood and Cognition
 Type 2 diabetes and obesity can cause cognitive impairment and accelerated brain aging, increasing risk for dementia. Studies in patients with type 2 diabetes have shown cerebral atrophy and neuropsychological dysfunction affecting a wide range of cognitive functions, including significantly reducing motor function, executive function, processing speed, verbal memory, and visual memory with only a small detriment to attention/concentration. There’s also a link between type 2 diabetes and depressive illness, each increasing the risk of the other.
Type 2 diabetes and obesity can cause cognitive impairment and accelerated brain aging, increasing risk for dementia. Studies in patients with type 2 diabetes have shown cerebral atrophy and neuropsychological dysfunction affecting a wide range of cognitive functions, including significantly reducing motor function, executive function, processing speed, verbal memory, and visual memory with only a small detriment to attention/concentration. There’s also a link between type 2 diabetes and depressive illness, each increasing the risk of the other.
While ketogenic diets have shown therapeutic promise in a wide range of neurodegenerative and neurologic diseases, including reducing seizures, improving memory, improving balance, improving cognition, and improving mood scores. There’s evidence that ketogenic diets can stabilize mood in bipolar disease and reduce or even prevent developmental delay in GLUT1 deficiency syndrome. Even in the case of mild cognitive impairment in the elderly, dietary ketosis can improve verbal memory performance. Because altered glucose metabolism and/or insulin resistance in the brain is implicated in these conditions, it makes sense that supplying ketones as an alternative energy source can ameliorate symptoms. However, there’s emerging evidence that ketogenic diets don’t have the same effect on the healthy brain.
In a study of cognitive performance in young, healthy men following a short-term ketogenic diet, participants showed significantly reduced focused concentration, memory retrieval speed, and speed of visual information processing. In another study of overweight women following a longer-term ketogenic diet, participants scored markedly lower on a neuropsychological test that requires higher order mental processing and flexibility (called the trail making task). Rodent studies have also shown detriment of ketogenic diets to cognition, with immature rats after status epilepticus having a reduction in seizures during dietary ketosis but also impaired visual-spatial learning and memory. Studies of low-carb, high-protein (nonketogenic) diets do not show reduced cognitive performance.
Studies in epileptic children have shown a trend towards mood problems and worsening psychosocial adjustment, although more data is definitely needed. In one study, overweight, sedentary adults were asked to walk for 90 minutes at 50-79% of their predicted maximum heart rate after following a ketogenic diet for two weeks. Participants had higher fatigue and total mood disturbance scores following exercise than calorie-matched controls, with overall reduced desire to exercise. Interestingly, tiredness and mood directly correlated with measured ketones.
Hormone Imbalance
 The relationship between insulin and hormones can best be summarized as: It’s complicated. That’s why it’s no surprise that effects of insulin resistance or ketogenic diets also have complicated effects on hormone systems.
The relationship between insulin and hormones can best be summarized as: It’s complicated. That’s why it’s no surprise that effects of insulin resistance or ketogenic diets also have complicated effects on hormone systems.
Insulin resistance is associated with polycystic ovary syndrome (PCOS), a condition characterized by high testosterone and anovulatory infertility, along with weight gain, acne, body hair, and depression. And, high testosterone in women can contribute to insulin resistance, creating a vicious cycle. Because the system is so complex though, insulin resistance may also be associated with low testosterone in women. In men, insulin resistance is associated with low testosterone levels, which decreases libido, strength, muscle mass, and bone density while increasing visceral fat deposition, but low testosterone reduces insulin sensitivity in men, again creating a vicious cycle.
In male athletes, ketogenic diets resulted in significantly increased testosterone levels and in women with PCOS, ketogenic diets improved symptoms by decreasing testosterone. In young, healthy rats, a ketogenic diet suppressed sex hormones, including metabolites of progesterone and testosterone. And, a significant percentage of young women following long-term ketogenic diets for epilepsy report amenorrhea (loss of menses). It’s likely the case that whether or not ketogenic diets are beneficial or harmful to hormone systems is very situation dependent.
Inflammation and Infection
 While inflammation is a hallmark of insulin resistance and observed in prediabetes, type 2 diabetes, obesity and metabolic syndrome, we also see increased susceptibility to infection in type 2 diabetes with protracted recovery time. This effect of increased inflammation but decreased immune function is seen in some studies of very low carbohydrate or ketogenic diets, although the need for more studies investigating specific effects on immune cell subsets is definitely needed.
While inflammation is a hallmark of insulin resistance and observed in prediabetes, type 2 diabetes, obesity and metabolic syndrome, we also see increased susceptibility to infection in type 2 diabetes with protracted recovery time. This effect of increased inflammation but decreased immune function is seen in some studies of very low carbohydrate or ketogenic diets, although the need for more studies investigating specific effects on immune cell subsets is definitely needed.
While a recent study in children with epilepsy showed that 10 days of a ketogenic diet reduced Th17 cell activity while increasing regulatory T cell activity (but not to normal levels), most studies evaluating effect of very low carbohydrate and ketogenic diets on inflammation don’t confirm this observation. One recent study showed an increase in C-reactive protein (a blood borne marker of inflammation) during weight loss with a low-carb, high-fat but not a high-carb diet. Another study in patients with rheumatoid arthritis showed no benefit to the cytokine IL-6 nor to T cell numbers or activation with a ketogenic diet. And in an animal study of malignant glioma, a ketogenic diet increased the numbers and activation of CD4+ T cells (especially Th1) while decreasing the population of regulatory T cells. Long-term studies of ketogenic diets in children and adolescents with epilepsy show a trend towards reduced white blood cells counts and neutrophil counts, while also reporting increased susceptibility to serious infection as an adverse reaction to ketogenic diets, affecting 10 to 15% of study participants.
Insulin Sensitivity Doesn’t Mean Low Carb
Maintaining insulin sensitivity is essential for health, but avoiding or drastically reducing carbohydrate intake isn’t necessary. Keeping blood sugar levels within a healthy range is easy if we limit ourselves to whole-food sources of carbohydrates (like starchy root vegetables and whole fruits) and eat them as part of a meal that includes animal protein and nonstarchy high-fiber veggies (like kale or broccoli). For those with insulin resistance or diabetes, measuring carbohydrate portions and keeping track of post-meal glucose levels is still advisable.
Insulin sensitivity is also inextricably tied to how much sleep we get, how stressed we are and how active we are. In fact, studies show that one night of poor sleep causes higher insulin resistance than 6 months of bad diet. That means that dialing in lifestyle factors is a necessity for restoring and maintaining insulin sensitivity.
And while ketogenic diets certainly have therapeutic value in neurodegenerative and neurologic diseases, it’s not the only show in town when it comes to weight loss, reversing type 2 diabetes, reducing cardiovascular disease risk factors, or lowering cancer risk. In fact, scientific studies confirm that a standard Paleo diet that embraces vegetable and fruit consumption is beneficial for all of these situations! See Paleo Diet Clinical Trials and Studies
How Many Carbs Should I Eat?
Most biological systems are subject to U-shaped response curves, meaning that either too little or too much causes problems. This type of response applies to how active we are, how much sun exposure we get, stress levels, nutrient status, hormone regulation, neurotransmitter regulation, immune response, temperature regulation, pH balance, a variety of external stimuli… Our bodies really seem to thrive under “happy medium” circumstances, and carbohydrates are no exception (see How many carbs should you eat?). So, what does happy medium translate to in terms of carbohydrate intake?
When optimizing carbohydrate intake, it’s important to avoid the effects of eating too little of any macronutrient (not just carbohydrates, but also fat and protein). Inadequate fat can decrease our absorption of vitamins A, D, E, and K, as well as deprive us of essential fatty acids. Inadequate protein can reduce the preservation of lean muscle tissue, reduce our immune function, negatively affect our bone mineral density, and cause a host of other problems related to insufficient intakes of essential amino acids. Too few carbohydrate can mean an insufficiency of fiber a well as other micronutrients abundant in vegetables and fruits, including: vitamin C, most B vitamins, vitamin K, potassium, magnesium, chromium, and a variety of phytochemicals including polyphenols, chlorophyll, carotenoids, isothiocyanates, and organosulfur compounds, all of which play various roles in disease prevention. The solution? We can balance our macronutrients by eating moderate amounts of carbohydrate, fat, and protein, rather than excessive or extremely limited levels of any single one.
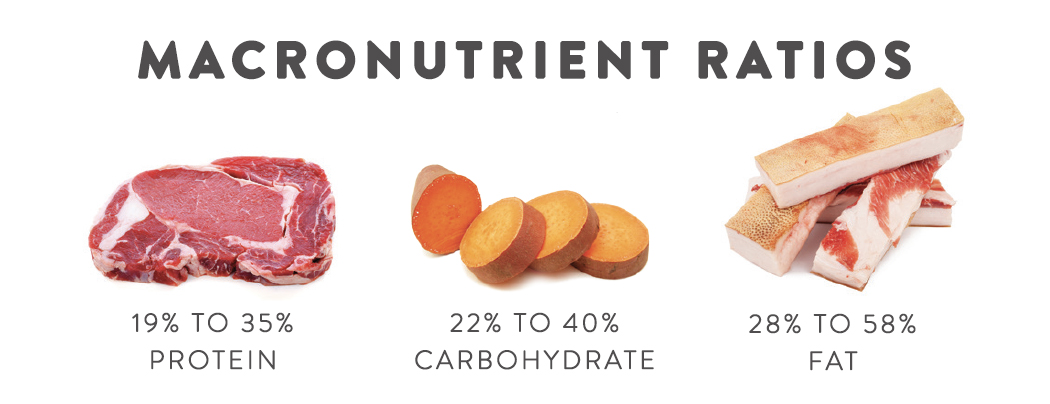
The majority of hunter-gatherer populations ate between 22 and 40% of their calories as carbohydrates, 19 to 35% protein, and 28 to 58% fat. That translates to 110 to 200 grams of total carbohydrates per day on a 2000-calorie diet. This is lower than the Accepted Macronutrient Distribution Range for carbohydrates set by the Food and Nutrition Board of the Institute of Medicine (which recommends 45 to 65% of calories from carbohydrates), but provided that our carbohydrates are coming from whole fruits and vegetables, the 100 to 200 carbohydrate gram range is likely adequate from a fiber consumption standpoint (USDA dietary guidelines are 25 grams daily for women and 30 to 38 grams daily for men) and from a micronutrient intake standpoint. Most healthcare providers and scientists would classify this level as moderate carbohydrate consumption.
For diagnosed type 2 diabetics, it is important to monitor blood sugar levels (as well as blood levels of HbA1C) and measure carbohydrate servings with each meal (this aspect of the American Diabetes Association guidelines are on point). It’s also important to note that the Paleo diet has been pitted against the American Diabetes Association dietary guidelines and shown to be superior in terms of blood sugar regulation and restoration of insulin sensitivity. See Paleo Diet Clinical Trials and Studies. There are also some supplements and nutrients that can help regulate insulin production and sensitivity, including chromium, berberine and inositol, however it’s important to always discuss supplements and medications with a qualified healthcare professional before taking or discontinuing them (see also 8 Nutrients for Leaky Gut).
Lifestyle changes are also critical for diabetes care and management and for restoration and maintenance of insulin sensitivity. See 3 Ways to Regulate Insulin that Have Nothing to Do With Food.
Citations
Abdulla H, Smith K, Atherton PJ, Idris I. Role of insulin in the regulation of human skeletal muscle protein synthesis and breakdown: a systematic review and meta-analysis. Diabetologia. 2016 Jan;59(1):44-55. doi: 10.1007/s00125-015-3751-0. Epub 2015 Sep 24.
Agnihothri RV, Courville AB, Linderman JD, et al. Moderate Weight Loss Is Sufficient to Affect Thyroid Hormone Homeostasis and Inhibit Its Peripheral Conversion. Thyroid. 2014;24(1):19-26. doi:10.1089/thy.2013.0055.
Amer J, Salhab A, Noureddin M, Doron S, Abu-Tair L, Ghantous R, Mahamid M, Safadi R. Insulin signaling as a potential natural killer cell checkpoint in fatty liver disease. Hepatol Commun. 2018 Feb 14;2(3):285-298. doi: 10.1002/hep4.1146. eCollection 2018 Mar.
Arneth B. Activation of CD4+ and CD8+ T-lymphocytes by insulin and GAD in patients with type 1 or 2 diabetes mellitus. Endocr Connect. 2017 Nov;6(8):758-765. doi: 10.1530/EC-17-0230. Epub 2017 Oct 6.
Arneth BM. Activation of CD4 and CD8 T cell receptors and regulatory T cells in response to human proteins. PeerJ. 2018 Mar 9;6:e4462. doi: 10.7717/peerj.4462. eCollection 2018.
Bergqvist AG, Schall JI, Stallings VA, Zemel BS. Progressive bone mineral content loss in children with intractable epilepsy treated with the ketogenic diet. Am J Clin Nutr. 2008 Dec;88(6):1678-84. doi: 10.3945/ajcn.2008.26099.
Bertoli, S., Trentani, C., Ferraris, C., De Giorgis, V., Veggiotti, P., Tagliabue, A. Long-term effects of a ketogenic diet on body composition and bone mineralization in GLUT-1 deficiency syndrome: a case series. Nutrition. 2014 Jun;30(6):726-8. doi: 10.1016/j.nut.2014.01.005. Epub 2014 Jan 29.
Brooks DC, Bessey PQ, Black PR, Aoki TT, Wilmore DW. Insulin stimulates branched chain amino acid uptake and diminishes nitrogen flux from skeletal muscle of injured patients. J Surg Res. 1986 Apr;40(4):395-405.
Cervenka MC, Henry-Barron BJ, Kossoff EH. Is there a role for diet monotherapy in adult epilepsy? Epilepsy Behav Case Rep. 2016 Sep 20;7:6-9. doi: 10.1016/j.ebcr.2016.09.005. eCollection 2017.
Chang CK, Borer K, Lin PJ. Low-Carbohydrate-High-Fat Diet: Can it Help Exercise Performance? J Hum Kinet. 2017 Mar 12;56:81-92. doi: 10.1515/hukin-2017-0025. eCollection 2017 Feb.
Chrysohoou C, Panagiotakos D, Pitsavos C, Siasos G, Oikonomou E, Varlas J, Patialiakas A, Lazaros G, Psaltopoulou T, Zaromitidou M, Kourkouti P, Tousoulis D, Stefanadis C. Low total testosterone levels are associated with the metabolic syndrome in elderly men: the role of body weight, lipids, insulin resistance, and inflammation; the Ikaria study. Rev Diabet Stud. 2013 Spring;10(1):27-38. doi: 10.1900/RDS.2013.10.27. Epub 2013 May 10.
Coppola G, Veggiotti P, Cusmai R, Bertoli S, Cardinali S, Dionisi-Vici C, Elia M, Lispi ML, Sarnelli C, Tagliabue A, Toraldo C, Pascotto A. The ketogenic diet in children, adolescents and young adults with refractory epilepsy: an Italian multicentric experience. Epilepsy Res. 2002 Feb;48(3):221-7.
Corbould A. Effects of androgens on insulin action in women: is androgen excess a component of female metabolic syndrome? Diabetes Metab Res Rev. 2008 Oct;24(7):520-32. doi: 10.1002/dmrr.872.
Cordain L, Miller JB, Eaton SB, Mann N, Holt SH, Speth JD. Plant-animal subsistence ratios and macronutrient energy estimations in worldwide hunter-gatherer diets. Am J Clin Nutr. 2000 Mar;71(3):682-92.
Costa Rosa LF, Safi DA, Cury Y, Curi R. The effect of insulin on macrophage metabolism and function. Cell Biochem Funct. 1996 Mar;14(1):33-42.
Daka B, Rosen T, Jansson PA, Råstam L, Larsson CA, Lindblad U. Inverse association between serum insulin and sex hormone-binding globulin in a population survey in Sweden. Endocr Connect. 2012 Nov 19;2(1):18-22. doi: 10.1530/EC-12-0057. Print 2013 Mar 1.
Dimitriadis G, Mitrou P, Lambadiari V, Maratou E, Raptis SA. Insulin effects in muscle and adipose tissue. Diabetes Res Clin Pract. 2011 Aug;93 Suppl 1:S52-9. doi: 10.1016/S0168-8227(11)70014-6.
Drigo RA, Fonseca TL, Werneck-de-Castro JPS, Bianco AC. Role of the type 2 iodothyronine deiodinase (D2) in the control of thyroid hormone signaling. Biochimica et biophysica acta. 2013;1830(7):3956-3964. doi:10.1016/j.bbagen.2012.08.019.
Eaton SB & Konner M. “Paleolithic nutrition. A consideration of its nature and current implications.” N Engl J Med. 1985 Jan 31;312(5):283-9.
Elia M, Klepper J, Leiendecker B, Hartmann H. Ketogenic Diets in the Treatment of Epilepsy. Curr Pharm Des. 2017;23(37):5691-5701. doi: 10.2174/1381612823666170809101517.
Fischer HJ, Sie C, Schumann E, Witte AK, Dressel R, van den Brandt J, Reichardt HM. The Insulin Receptor Plays a Critical Role in T Cell Function and Adaptive Immunity. J Immunol. 2017 Mar 1;198(5):1910-1920. doi: 10.4049/jimmunol.1601011. Epub 2017 Jan 23.
Fraser DA, Thoen J, Bondhus S, Haugen M, Reseland JE, Djøseland O, Førre O, Kjeldsen-Kragh J. Reduction in serum leptin and IGF-1 but preserved T-lymphocyte numbers and activation after a ketogenic diet in rheumatoid arthritis patients. Clin Exp Rheumatol. 2000 Mar-Apr;18(2):209-14.
Fulzele K, Clemens TL. Novel functions for insulin in bone. Bone. 2012 Feb;50(2):452-6. doi: 10.1016/j.bone.2011.06.018. Epub 2011 Jun 24.
Gross LS, et al, Increased consumption of refined carbohydrates and the epidemic of type 2 diabetes in the United States: an ecologic assessment. Am J Clin Nutr. 2004 May;79(5):774-9.
Hall KD, Chen KY, Guo J, Lam YY, Leibel RL, Mayer LE, Reitman ML, Rosenbaum M, Smith SR, Walsh BT, Ravussin E. Energy expenditure and body composition changes after an isocaloric ketogenic diet in overweight and obese men. Am J Clin Nutr. 2016 Aug;104(2):324-33. doi: 10.3945/ajcn.116.133561. Epub 2016 Jul 6.
Hall KD, Chung ST. Low-carbohydrate diets for the treatment of obesity and type 2 diabetes. Curr Opin Clin Nutr Metab Care. 2018 Apr 18. doi: 10.1097/MCO.0000000000000470.
Hallböök T, Ji S, Maudsley S, Martin B. The effects of the ketogenic diet on behavior and cognition. Epilepsy Res. 2012 Jul;100(3):304-9. doi: 10.1016/j.eplepsyres.2011.04.017. Epub 2011 Aug 27.
Han JM, Patterson SJ, Speck M, Ehses JA, Levings MK. Insulin inhibits IL-10-mediated regulatory T cell function: implications for obesity. J Immunol. 2014 Jan 15;192(2):623-9. doi: 10.4049/jimmunol.1302181. Epub 2013 Dec 9.
Holloway CJ, Cochlin LE, Emmanuel Y, Murray A, Codreanu I, Edwards LM, Szmigielski C, Tyler DJ, Knight NS, Saxby BK, Lambert B, Thompson C, Neubauer S, Clarke K. A high-fat diet impairs cardiac high-energy phosphate metabolism and cognitive function in healthy human subjects. Am J Clin Nutr. 2011 Apr;93(4):748-55. doi: 10.3945/ajcn.110.002758. Epub 2011 Jan 26.
Husemoen LL, Glümer C, Lau C, Pisinger C, Mørch LS, Linneberg A. Association of obesity and insulin resistance with asthma and aeroallergen sensitization. Allergy. 2008 May;63(5):575-82. doi: 10.1111/j.1398-9995.2007.01613.x.
Hylla S, et al. “Effects of resistant starch on the colon in healthy volunteers: possible implications for cancer prevention.” Am J Clin Nutr. 1998 Jan;67(1):136-42.
Jin HY. Prevalence of subclinical hypothyroidism in obese children or adolescents and association between thyroid hormone and the components of metabolic syndrome. J Paediatr Child Health. 2018 May 16. doi: 10.1111/jpc.13926
Johnston KL, et al. “Resistant starch improves insulin sensitivity in metabolic syndrome.” Diabet Med. 2010;27:391-397.
Kang HC, Chung DE, Kim DW, Kim HD. Early- and late-onset complications of the ketogenic diet for intractable epilepsy. Epilepsia. 2004 Sep;45(9):1116-23.
Kang HC, Kim YJ, Kim DW, Kim HD. Efficacy and safety of the ketogenic diet for intractable childhood epilepsy: Korean multicentric experience. Epilepsia. 2005 Feb;46(2):272-9.
Kapadia KB, Bhatt PA, Shah JS. Association between altered thyroid state and insulin resistance. Journal of Pharmacology & Pharmacotherapeutics. 2012;3(2):156-160. doi:10.4103/0976-500X.95517.
Kose E, Guzel O, Demir K, Arslan N. Changes of thyroid hormonal status in patients receiving ketogenic diet due to intractable epilepsy. J Pediatr Endocrinol Metab. 2017 Apr 1;30(4):411-416. doi: 10.1515/jpem-2016-0281.
Krikorian R, Shidler MD, Dangelo K, Couch SC, Benoit SC, Clegg DJ. Dietary ketosis enhances memory in mild cognitive impairment. Neurobiol Aging. 2012 Feb;33(2):425.e19-27. doi: 10.1016/j.neurobiolaging.2010.10.006. Epub 2010 Dec 3.
Lager I. The insulin-antagonistic effect of the counterregulatory hormones. J Intern Med Suppl. 1991;735:41-7.
Lambrechts DA, Bovens MJ, de la Parra NM, Hendriksen JG, Aldenkamp AP, Majoie MJ. Ketogenic diet effects on cognition, mood, and psychosocial adjustment in children. Acta Neurol Scand. 2013 Feb;127(2):103-8. doi: 10.1111/j.1600-0404.2012.01686.x. Epub 2012 Jun 12.
Laron Z. Insulin and the brain. Arch Physiol Biochem. 2009 May;115(2):112-6. doi: 10.1080/13813450902949012.
Lu H, Huang D, Yao K, Li C, Chang S, Dai D, Sun A, Zou Y, Qian J, Ge J. Insulin enhances dendritic cell maturation and scavenger receptor-mediated uptake of oxidised low-density lipoprotein, J Diab Comp 2015 29(4):465-71 doi: 10.1016/j.jdiacomp.2015.03.005.
Lussier DM, Woolf EC, Johnson JL, Brooks KS, Blattman JN, Scheck AC. Enhanced immunity in a mouse model of malignant glioma is mediated by a therapeutic ketogenic diet. BMC Cancer. 2016 May 13;16:310. doi: 10.1186/s12885-016-2337-7.
Makris A, Darcey VL, Rosenbaum DL, Komaroff E, Vander Veur SS, Collins BN, Klein S, Wyatt HR, Foster GD. Similar effects on cognitive performance during high- and low-carbohydrate obesity treatment. Nutr Diabetes. 2013 Sep 23;3:e89. doi: 10.1038/nutd.2013.29.
Martinez-deMena R, Obregón M. Insulin increases the adrenergic stimulation of 5′ deiodinase activity and mRNA expression in rat brown adipocytes; role of MAPK and PI3K. J Mol Endocrinol. 2005 Feb;34(1):139-51.
Martinez-Sanchez ME, Hiriart M, Alvarez-Buylla ER. The CD4+ T cell regulatory network mediates inflammatory responses during acute hyperinsulinemia: a simulation study. BMC Syst Biol. 2017 Jun 26;11(1):64. doi: 10.1186/s12918-017-0436-y.
Mavropoulos JC, Yancy WS, Hepburn J, Westman EC. The effects of a low-carbohydrate, ketogenic diet on the polycystic ovary syndrome: a pilot study. Nutr Metab (Lond). 2005 Dec 16;2:35.
Milne NT, Bucks RS, Davis WA, Davis TME, Pierson R, Starkstein SE, Bruce DG. Hippocampal atrophy, asymmetry, and cognition in type 2 diabetes mellitus. Brain Behav. 2017 Dec 22;8(1):e00741. doi: 10.1002/brb3.741. eCollection 2018 Jan.
Nakao R, Shimba S, Oishi K. Ketogenic diet induces expression of the muscle circadian gene Slc25a25 via neural pathway that might be involved in muscle thermogenesis. Sci Rep. 2017 Jun 6;7(1):2885. doi: 10.1038/s41598-017-03119-8.
Nguyen TTL, Chan LC, Borreginne K, Kale RP, Hu C, Tye SJ. A Review of Brain Insulin Signaling in Mood Disorders: From Biomarker to Clinical Target. Neurosci Biobehav Rev. 2018 May 11. pii: S0149-7634(17)30788-1. doi: 10.1016/j.neubiorev.2018.05.014.
Ni FF, Li CR, Liao JX, Wang GB, Lin SF, Xia Y, Wen JL. The effects of ketogenic diet on the Th17/Treg cells imbalance in patients with intractable childhood epilepsy. Seizure. 2016 May;38:17-22. doi: 10.1016/j.seizure.2016.03.006. Epub 2016 Mar 22.
Nici J, Hom J. Neuropsychological function in type 2 diabetes mellitus. Appl Neuropsychol Adult. 2018 May 4:1-9. doi: 10.1080/23279095.2018.1455683.
Nilsson AC, et al. “Including indigestible carbohydrates in the evening meal of healthy subjects improves glucose tolerance, lowers inflammatory markers, and increases satiety after a subsequent standardized breakfast.” J Nutr. 2008;138:732-739.
Nilsson J, Ericsson M, Joibari MM, Anderson F, Carlsson L, Nilsson SK, Sjödin A, Burén J2. A low-carbohydrate high-fat diet decreases lean mass and impairs cardiac function in pair-fed female C57BL/6J mice. Nutr Metab (Lond). 2016 Nov 15;13:79. doi: 10.1186/s12986-016-0132-8. eCollection 2016.
Palta P, Schneider AL, Biessels GJ, Touradji P, Hill-Briggs F. Magnitude of cognitive dysfunction in adults with type 2 diabetes: a meta-analysis of six cognitive domains and the most frequently reported neuropsychological tests within domains. J Int Neuropsychol Soc. 2014 Mar;20(3):278-91. doi: 10.1017/S1355617713001483. Epub 2014 Feb 20.
Paoli A. Ketogenic diet for obesity: friend or foe? Int J Environ Res Public Health. 2014 Feb 19;11(2):2092-107. doi: 10.3390/ijerph110202092.
Pasquali R, Casimirri F, De Iasio R, Mesini P, Boschi S, Chierici R, Flamia R, Biscotti M, Vicennati V. Insulin regulates testosterone and sex hormone-binding globulin concentrations in adult normal weight and obese men. J Clin Endocrinol Metab. 1995 Feb;80(2):654-8.
Pramojanee SN, Phimphilai M, Chattipakorn N, Chattipakorn SC. Possible roles of insulin signaling in osteoblasts. Endocr Res. 2014;39(4):144-51. doi: 10.3109/07435800.2013.879168. Epub 2014 Mar 28.
Pscherer S, Sandmann GH, Ehnert S, Nussler AK, Stöckle U, Freude T. Delayed Fracture Healing in Diabetics with Distal Radius Fractures. Acta Chir Orthop Traumatol Cech. 2017;84(1):24-29.
Ramm-Pettersen A, Stabell KE, Nakken KO, Selmer KK. Does ketogenic diet improve cognitive function in patients with GLUT1-DS? A 6- to 17-month follow-up study. Epilepsy Behav. 2014 Oct;39:111-5. doi: 10.1016/j.yebeh.2014.08.015. Epub 2014 Sep 18.
Rankin JW, Turpyn AD. Low carbohydrate, high fat diet increases C-reactive protein during weight loss. J Am Coll Nutr. 2007 Apr;26(2):163-9.
Rao PM, Kelly DM, Jones TH. Testosterone and insulin resistance in the metabolic syndrome and T2DM in men. Nat Rev Endocrinol. 2013 Aug;9(8):479-93. doi: 10.1038/nrendo.2013.122. Epub 2013 Jun 25.
Reagan LP. Insulin signaling effects on memory and mood. Curr Opin Pharmacol. 2007 Dec;7(6):633-7. Epub 2007 Nov 26.
Riddle RC, Clemens TL. Insulin, osteoblasts, and energy metabolism: why bone counts calories. The Journal of Clinical Investigation. 2014;124(4):1465-1467. doi:10.1172/JCI75554.
Sanches CP, Vianna AGD, Barreto FC. The impact of type 2 diabetes on bone metabolism. Diabetol Metab Syndr. 2017 Oct 19;9:85. doi: 10.1186/s13098-017-0278-1. eCollection 2017.
Schreck KC, Lwin M, Strowd RE, Henry-Barron BJ, Blakeley JO, Cervenka MC. Effect of ketogenic diets on leukocyte counts in patients with epilepsy. Nutr Neurosci. 2017 Dec 18:1-6. doi: 10.1080/1028415X.2017.1416740. [Epub ahead of print]
Shaw JA, Shetty P, Burns KD, Fergusson D, Knoll GA. C-peptide as a Therapy for Kidney Disease: A Systematic Review and Meta-Analysis. PLoS One. 2015 May 20;10(5):e0127439. doi: 10.1371/journal.pone.0127439. eCollection 2015.
Simm PJ, Bicknell-Royle J, Lawrie J, Nation J, Draffin K, Stewart KG, Cameron FJ, Scheffer IE, Mackay MT. The effect of the ketogenic diet on the developing skeleton. Epilepsy Res. 2017 Oct;136:62-66. doi: 10.1016/j.eplepsyres.2017.07.014. Epub 2017 Jul 26.
Souteiro P, Belo S, Oliveira SC, Neves JS, Magalhães D, Pedro J, Bettencourt-Silva R, Costa MM, Varela A, Queirós J, Freitas P, Carvalho D; AMTCO Group. Insulin resistance and sex hormone-binding globulin are independently correlated with low free testosterone levels in obese males. Andrologia. 2018 May 9:e13035. doi: 10.1111/and.13035. [Epub ahead of print]
Spielman LJ, Bahniwal M, Little JP, Walker DG, Klegeris A. Insulin Modulates In Vitro Secretion of Cytokines and Cytotoxins by Human Glial Cells. Curr Alzheimer Res. 2015;12(7):684-93.
Srikanthan P, Karlamangla AS. Muscle mass index as a predictor of longevity in older adults. Am J Med. 2014 Jun;127(6):547-53. doi: 10.1016/j.amjmed.2014.02.007. Epub 2014 Feb 18.
Strain G, Zumoff B, Rosner W, Pi-Sunyer X. The relationship between serum levels of insulin and sex hormone-binding globulin in men: the effect of weight loss. J Clin Endocrinol Metab. 1994 Oct;79(4):1173-6.
Ströhle A & Hahn A. “Diets of modern hunter-gatherers vary substantially in their carbohydrate content depending on ecoenvironments: results from an ethnographic analysis.” Nutr Res. 2011 Jun;31(6):429-35.
Suba Z. Interplay between insulin resistance and estrogen deficiency as co- activators in carcinogenesis. Pathol Oncol Res. 2012 Apr;18(2):123-33. doi: 10.1007/s12253-011-9466-8. Epub 2011 Oct 9.
Sunahara KK, Sannomiya P, Martins JO. Briefs on insulin and innate immune response. Cell Physiol Biochem. 2012;29(1-2):1-8. doi: 10.1159/000337579. Epub 2012 Mar 1.
Thienel M, Wilhelm I, Benedict C, Born J, Hallschmid M. Intranasal insulin decreases circulating cortisol concentrations during early sleep in elderly humans. Neurobiol Aging. 2017 Jun;54:170-174. doi: 10.1016/j.neurobiolaging.2017.03.006. Epub 2017 Mar 14.
Tinsley GM, Willoughby DS. Fat-Free Mass Changes During Ketogenic Diets and the Potential Role of Resistance Training. Int J Sport Nutr Exerc Metab. 2016 Feb;26(1):78-92. doi: 10.1123/ijsnem.2015-0070. Epub 2015 Aug 12.
Trierweiler H, Kisielewicz G, Hoffmann Jonasson T, Rasmussen Petterle R, Aguiar Moreira C, Zeghbi Cochenski Borba V. Sarcopenia: a chronic complication of type 2 diabetes mellitus. Diabetol Metab Syndr. 2018 Apr 3;10:25. doi: 10.1186/s13098-018-0326-5. eCollection 2018.
Vazquez JA, Adibi SA. Protein sparing during treatment of obesity: ketogenic versus nonketogenic very low calorie diet. Metabolism. 1992 Apr;41(4):406-14.
Wahren J, Larsson C. C-peptide: new findings and therapeutic possibilities. Diabetes Res Clin Pract. 2015 Mar;107(3):309-19. doi: 10.1016/j.diabres.2015.01.016. Epub 2015 Jan 21.
White AM, Johnston CS, Swan PD, Tjonn SL, Sears B. Blood ketones are directly related to fatigue and perceived effort during exercise in overweight adults adhering to low-carbohydrate diets for weight loss: a pilot study. J Am Diet Assoc. 2007 Oct;107(10):1792-6.
Wilson JM, Lowery RP, Roberts MD, Sharp MH, Joy JM, Shields KA, Partl J, Volek JS, D’Agostino D. The Effects of Ketogenic Dieting on Body Composition, Strength, Power, and Hormonal Profiles in Resistance Training Males. J Strength Cond Res. 2017 Apr 7. doi: 10.1519/JSC.0000000000001935.
Wing RR, Vazquez JA, Ryan CM 1995 Cognitive effects of ketogenic weight-reducing diets. Int J Obes Relat Metab Disord 19:811–816
Wroble KA, Trott MN, Schweitzer GG, Rahman RS, Kelly PV, Weiss EP. Low-carbohydrate, ketogenic diet impairs anaerobic exercise performance in exercise-trained women and men: a randomized-sequence crossover trial. J Sports Med Phys Fitness. 2018 Apr 4. doi: 10.23736/S0022-4707.18.08318-4.
Yosten GLC, Maric-Bilkan C, Luppi P, Wahren J. Physiological effects and therapeutic potential of proinsulin C-peptide. Am J Physiol Endocrinol Metab. 2014 Dec 1; 307(11): E955–E968. Published online 2014 Sep 23. doi: 10.1152/ajpendo.00130.2014
Zhang B, Wang J, Shen S, Liu J, Sun J, Gu T, Ye X, Zhu D, Bi Y. Association of Androgen Excess with Glucose Intolerance in Women with Polycystic Ovary Syndrome. Biomed Res Int. 2018 Mar 8;2018:6869705. doi: 10.1155/2018/6869705. eCollection 2018.
Zhao Q, Stafstrom CE, Fu DD, Hu Y, Holmes GL. Detrimental effects of the ketogenic diet on cognitive function in rats. Pediatr Res. 2004 Mar;55(3):498-506. Epub 2004 Jan 7
Zhao WQ, Alkon DL. Role of insulin and insulin receptor in learning and memory. Mol Cell Endocrinol. 2001 May 25;177(1-2):125-34.
Zhao WQ, Chen H, Quon MJ, Alkon DL. Insulin and the insulin receptor in experimental models of learning and memory. Eur J Pharmacol. 2004 Apr 19;490(1-3):71-81.