Table of Contents[Hide][Show]
- The Scientific Base for mRNA Vaccines
- Advantages of mRNA Vaccines: Speed of Manufacture
- Advantages of mRNA Vaccines: Safety
- Advantages of mRNA Vaccines: Efficacy without Adjuvants
- More Durable Immunity than Natural Infection
- Pfizer vs. Moderna (vs. J&J)
- Post Inoculation Side Effects – What to Expect
- Busting Covid-19 Vaccine Myths
- Benefits to Long Covid
- What Can You Do After Getting Vaccinated?
- Who Should Get Vaccinated?
- Citations
There are many vaccine myths amplified by the internet leading to vaccine hesitancy with the new covid-19 vaccines. And, I completely understand how tough it can be to vet and identify misinformation online, especially in these anxious and difficult times. You don’t need a doctorate degree in Medical Biophysics, like I have, to learn the science behind these vaccines and separate the facts from fiction. So, because I’ve spent something like 80 hours cumulative over the last five months reading through peer-reviewed published papers on these vaccines, I want to objectively share the science behind them with you, focusing on the two currently-approved mRNA vaccines, Pfizer/BioNTech and Moderna (but with some asides relevant to the adenovirus vector vaccines from Johnson & Johnson/Janssen and AstraZeneca/Oxford). My goal is to cut through the mis- and disinformation and give you the knowledge you need to make the best choice for you and your community.
Covid-19, the pandemic infectious disease caused by the novel coronavirus SARS-CoV-2, has directly caused at least 584,000 deaths in the USA (a case fatality rate of about 1.8%), and nearly 3.3 million deaths worldwide (a case fatality rate of about 3.5%). Variants of concern (slight mutations of the novel coronavirus that give it a competitive advantage) have emerged from areas of high community spread—they are more contagious and, in some cases, also have higher rates of severe and critical disease and higher mortality (see also Natural Approaches to Cold & Flu Season (and Covid-19!), Covid-19 and the Gut, and Covid-19 FAQ: Do Face Masks Even Work?).

Save 80% Off the Foundations of Health
Expand your health knowledge on a wide range of topics relevant to you, from how to evaluate scientific studies, to therapeutic diet and lifestyle, to leaky gut and gut microbiome health, to sustainable weight loss, and much more!!!

We have all had to adjust our lives in response to the pandemic, from wearing face masks and social distancing, to isolation and quarantine, to virtual school and work, to the economic impact felt in so many households (see Covid-19 FAQ: Do Face Masks Even Work?, How to Prepare for a Coronavirus Shutdown, TPV Podcast Episode 398: How We’re Coping with Quarantine, TWV Podcast Episode 410: How We’re Coping with Quarantine, Part 2, and TWV Podcast Episode 428 Quarantine Holidays). But, there is finally some good news: Scientists have delivered a pathway through to the other side of this pandemic by developing effective and safe vaccines in record time. And, while there are still logistical challenges to overcome in the vaccination effort (especially globally), one of the biggest hurdles here in the USA is vaccine hesitancy.
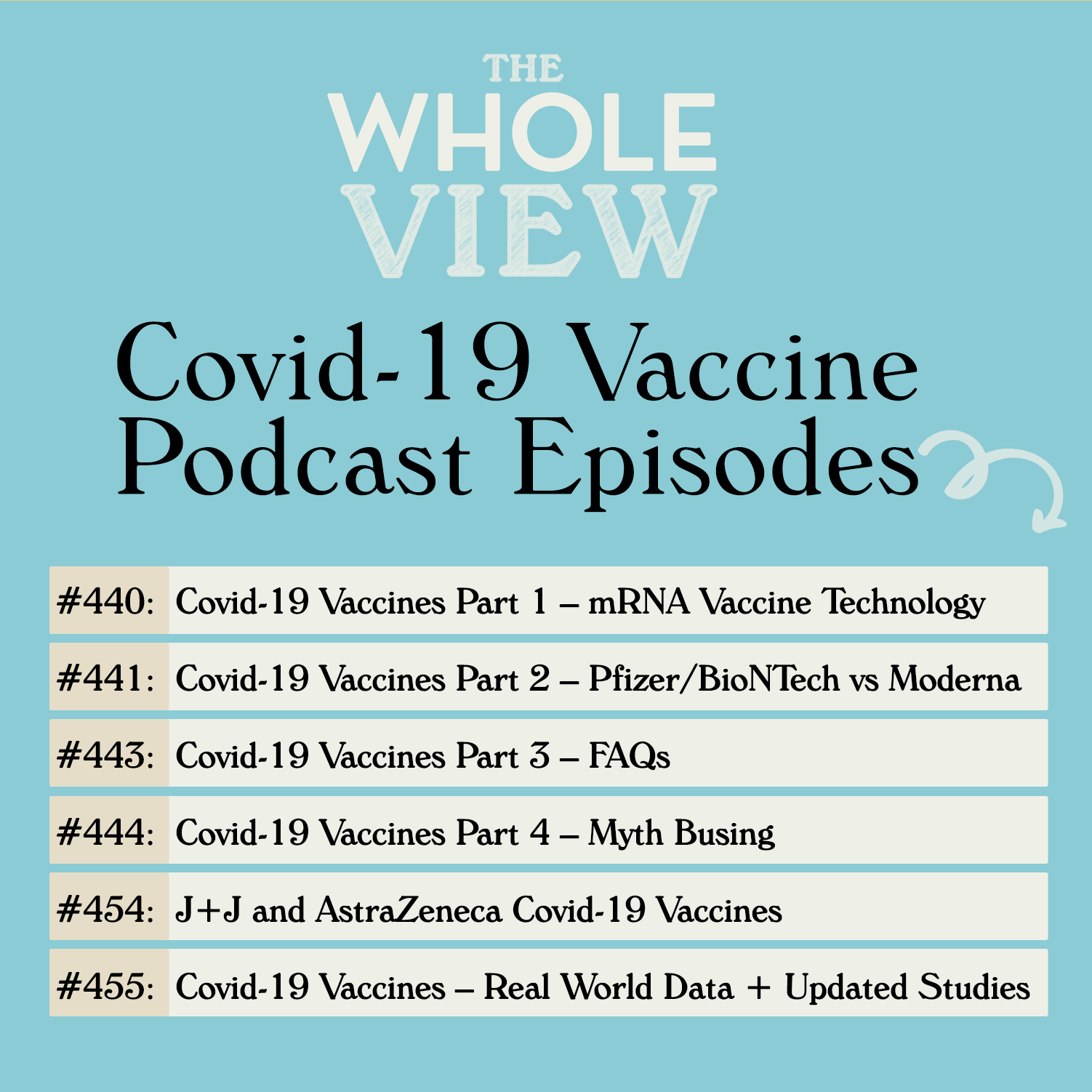
My most thorough resource for understanding the vaccines is my epic 6-part podcast series, so if you still have questions after reading this article, make sure to check these episodes out:
- TWV Podcast Episode 440: Covid-19 Vaccines Part 1 – mRNA Vaccine Technology In this episode, Stacy and I examined the history of vaccines, the very real statistics on vaccine-induced injury, and the advances that led to mRNA vaccine technology along with the inherent advantages of this platform.
- TWV Podcast Episode 441: Covid-19 Vaccines Part 2 – Pfizer/BioNTech vs Moderna In this episode, we looked at the safety and efficacy data from the phase 2/3 clinical trials for both the Pfizer/BioNTech and the Moderna covid-19 vaccines, including subgroup analysis.
- TWV Podcast Episode 443: Covid-19 Vaccines Part 3 – FAQs In this episode, we answered listener FAQ, including concerns about adverse events including autoimmune disease, fertility, and the current state of evidence in terms of safety concerns for pregnancy and children.
- TWV Podcast Episode 444: Covid-19 Vaccines Part 4 – Myth Busing In this episode, we talk about the myths surrounding antibody-enhanced infection, the for-profit argument, mRNA vs. DNA, traces of controversial tissue cells, and hidden foreign bodies. We also cover who should wait to get the vaccine and vaccine aftercare.
- TWV Podcast Episode 454: J&J and AstraZeneca Covid-19 Vaccines In this episode, we look at how adenovirus vaccines work and the safety and efficacy data from the phase 2/3 clinical trials for both the Johnson & Johnson & Janssen and the AstraZeneca/Oxford University vaccines, including a deep dive into immune thrombotic thrombocytopenia (what the news is reporting as a rare type of blood clot).
- TWV Podcast Episode 455: Covid-19 Vaccines – Real World Data and Updated Studies In this episode, we talk about new studies in pregnant and lactating women, breakthrough infections, updated vaccine studies for the second dose, variants of concern coverage by vaccines, monoclonal antibody therapies and menstruation irregularities.
- TWV Podcast Episode 468: The Delta Covid-19 Variant In this episode, we talk about the mutations that make the Delta variant so much more contagious, what that means for vaccine efficacy, and public health measures to protect ourselves and our communities.
- TWV Podcast Episode 473: Answering Listener Questions LIVE! In this live Q&A with our Patreon, we answered several questions related to the covid-19 Delta variant and the covid-19 vaccines.
- TWV Podcast Episode 492: The Omicron Variant of Covid-19 In this episode, we dive deep into the omicron variant of covid-19, including its mutations, where it likely came from (mice!), what makes it so transmissible, its symptoms and how they differ from previous variants, whether or not it’s actually more mild than other variants, how good of a job vaccines are doing at protecting us, and why this variant was able to displace the Delta variant.
- TWV Podcast Episode 493: How to Prepare for Omicron Covid-19 Infection In this episode, we cover latest science on the causes of long-covid, and the chances of other health problems caused by covid-19 like increased diabetes risk and long-term olfactory dysfunction. We also review the importance of vaccination and boosters, mask wearing and upgrading to N95 or KN95 masks, and continuing social distancing as first line protection against the Omicron variant. We then examine the links between nutrients, diet habits, and lifestyle and susceptibility to covid-19 infection, including vitamin D, vitamin C, zinc, magnesium, a veggie-forward diet, getting enough sleep on a consistent basis, and living an active lifestyle.
And, of course, these shows build on the covid-19 science discussed in:
- TPV Podcast Episode 394: Covid-19 In this episode, we address all things coronavirus, also known as covid-19. What exactly is this disease? What are the symptoms? How is it spread? And what can we do to reduce our risk of infection?
- TPV Podcast Episode 396: Covid-19 FAQ In this episode, we answer listeners’ questions on covid-19. What does the latest data say on what to expect? What are the biggest risk factors? And are there ways to minimize our risk or symptoms if we do get sick?
- TWV Podcast Episode 401: Covid-19 NEW FAQ In this episode, we answer even more listeners’ questions on covid-19. What is our way out? What about vaccine development? Do non-medical grade face masks really make a difference? Rapid testing? and reinfection?
- TWV Podcast Episode 412: Covid-19 FAQ, Part 3 In this episode, we talk about how quarantine is impacting our gut microbiome, if healthy habits and supplements can lower our risk of getting sick, and if science has advanced on what we know about antibodies and immunity.
- TWV Podcast Episode 425: Covid-19 FAQ Part 4 In this episode, we dive into even more coronavirus discussion, focusing on the long-term impacts of covid-19, the validity of reinfection cases, and the science behind covid-19 reactivation.
So, let’s start at the beginning, the amazing scientific advances over the last few decades that make the rapid (but not rushed) development of the covid-19 mRNA vaccines even possible!
The Scientific Base for mRNA Vaccines
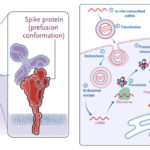
mRNA-based drug technology is extremely exciting. mRNA is the intermediate step between DNA instructions and production of the specific protein that DNA encodes. In our cells, DNA in the nucleus is first transcribed into mRNA, which is transported out of the nucleus and into the cytoplasm, where it is translated into protein by ribosomes in the cytoplasm or endoplasmic reticulum. While mRNA was first discovered in 1961, the initial proof-of-concept experiments showing the feasibility of mRNA-based drugs were performed in 1990. Cells can be transfected with mRNA by encapsulating it within a cationic (positively-charged) lipid envelope, which facilitates entrance into the cell via endocytosis and exit from the resulting endosome into the cytoplasm. There, the mRNA is translated into protein by the cell’s ribosomes.

There are many applications of this technology. In the case of mRNA vaccines like the new covid-19 vaccines from Pfizer/BioNTech and Moderna, mRNA encoding a subunit of the pathogen is delivered to cells, resulting in production by our own cells of an immunogenic foreign protein (antigen) that has no infectious capacity. This bypasses a common problem with traditional subunit vaccines, maintaining tertiary and quaternary protein structure throughout vaccine production, storage and administration. This technology has also been used in clinical trials to create personalized cancer vaccines, where mRNA for a mutated tumor protein is delivered to an area near the tumor, instructing the immune system to attack cancerous cells. Another application is protein replacement; for example, this technology has great promise for treating cystic fibrosis. Before 2020, over twenty mRNA-based candidate drugs had entered the clinical trial stage, including personalized cancer vaccines and vaccines for HIV, influenza, tuberculosis, RSV, and tick-borne encephalitis. Preclinical work on mRNA-based drugs for protein replacement included treatment for type 1 diabetes, autoimmune myocarditis, congenital lung disease, asthma and allergies.
Recent advances in mRNA-based drug technologies (including improvements to increase protein translation, modulate innate and adaptive immunogenicity, and improve mRNA delivery and halflife), in addition to coronavirus research following the SARS and MERS pandemics, set the stage for the rapid development of the covid-19 mRNA vaccines by both Pfizer/BioNTech and Moderna.
Advantages of mRNA Vaccines: Speed of Manufacture
In fact, one of the huge advantages of mRNA vaccines is their potential for rapid, inexpensive and scalable manufacturing.
For example, it took a mere 2 days after the virus genome was sequenced in January, 2020 for Moderna to create the mRNA sequence that produces a membrane-bound and prefusion confirmation stabilized version of the SARS-CoV-2 spike protein. Moderna was then able to ship its first vial of vaccine to NIH for trials 41 days after that. This short timeline wasn’t because the vaccine was “rushed” and no corners were cut, but instead this rapid development is thanks to the technology itself making mRNA vaccines extremely quick to design, develop and produce!
This awesome platform advantage also means that the covid-19 vaccine can be modified for new variants of concern or strains as they emerge. Newly published data shows that the Pfizer/BioNTech is about 90% effective against the more-contagious B.1.1.7 variant (first identified in the United Kingdom), but there’s some data indicating that neither the Pfizer/BioNTech nor the Moderna vaccine (nor natural immunity for that matter) will be as durable against the B.1.351 variant (first identified in South African) nor the P.1 variant (first identified in Brazil). To clarify, both the Pfizer/BioNTech and Moderna covid-19 vaccines offer protection against these variants of concern (after the second shot), but because neutralizing antibody binding to the virus variants is about 6-fold lower (but above threshold for protection), it’s expected that immunity won’t last as long against these variants. It’s also expected that the durability of protection against the B.1.617 (the variant fueling India’s current covid-19 crisis) will also be lower. Both Pfizer/BioNTech and Moderna are testing a third booster to improve coverage against the variants, and have released preliminary data showing efficacy.
Advantages of mRNA Vaccines: Safety

The second major advantage to mRNA vaccines is safety. First, because mRNA is non-infectious and non-integrating, there is zero potential risk of infection or insertional mutagenesis. That means that the mRNA vaccines do not and can not alter our DNA (and, for reference, neither do the adenovirus vector covid-19 vaccines, since adenoviruses lack the enzymatic machinery needed to alter DNA, see TWV Podcast Episode 454: J&J and AstraZeneca Covid-19 Vaccines). There is no immunogenic vector to be concerned about and no need for an adjuvant. Additionally, mRNA is degraded by normal cellular processes—its half-life ranges from 3 to 30 hours. (This is an awesome property for vaccines, but a challenge for protein replacement applications.)
Traditional vaccines carry with them a small, but nonzero, risk for vaccine-induced injury, including severe allergic reaction, encephalitis, Dawson disease, immune thrombocytopenic purpura, pneumonia, and Guillain-Barré syndrome. In most cases, the risk from natural infection is much greater than the risk from vaccination; for example, the risk of mild to severe immune thrombocytopenic purpura from the MMR vaccine is 1 in 40,000 but the risk from rubella infection is 1 in 3,000. Even in the case of Guillain-Barre syndrome, which is linked to 1 in 1.25 million influenza vaccinations, newer studies are showing that natural influenza infection has an even stronger association with Guillain-Barré, implying that the annual flu vaccination may actually decrease the overall incidence.
The results from the Pfizer/BioNTech and Moderna covid-19 vaccine phase 2/3 clinical trials indicate no increased risk of these kinds of serious adverse reactions, including no risk for new or worsening autoimmune disease. And while it was always possible that a serious adverse reaction could occur with a low enough frequency that it would not be detected until much larger numbers of vaccinations are administered, ongoing monitoring of vaccine side effects and adverse reactions have only turned up one low-frequency adverse event: serious allergic reactions to the covid-19 mRNA vaccines. This is thought to be caused by a rare allergy to polyethylene glycol (PEG), which is one of the four lipid nanoparticles in the lipid envelope of both mRNA vaccines. PEG can also be found in some other vaccines, many medications and laxatives. If you have a history of anaphylactic allergic reactions, you’ll be asked to stay for 30 minutes instead of 15 after your vaccination for monitoring, so medical treatment can be administered just in case.
As an aside, ongoing monitoring did turn up a rare adverse event to the adenovirus vector covid-19 vaccines, immune thrombotic thrombocytopenia (typically misrepresented as rare “blood clots” in the media). This is discussed in detail in TWV Podcast Episode 454: J&J and AstraZeneca Covid-19 Vaccines, which I encourage you to listen to, but it’s important to note here that detecting this rare adverse effect is exactly what community monitoring is for. The FDA pause on administering the Johnson & Johnson/Janssen vaccine allowed for education of vaccine recipient and healthcare providers. Immune thrombotic thrombocytopenia requires medical attention and is highly treatable, but the treatment is not the same as embolisms or thromboses. Also, make sure to sign up for v-safe when you do get your vaccine, so you can participate in ongoing monitoring—it’s really important!
While the original phase 2/3 clinical trials did not collect rigorous safety data in pregnant or lactating women, subsequent studies have shown no obvious safety signals to be concerned about. This is especially important because pregnant women are overall 3 to 3.5 times more likely to require ventilation and 70% more likely to die from covid-19 than their age and risk factor-matched controls, with advanced maternal age (AMA) women at even greater risk. Pregnant women aged 35–44 years are nearly four times more likely to require invasive ventilation during covid-19 infection, and have approximately double the mortality rate of nonpregnant women of the same age
A study of pregnant v-safe participants who received either the Pfizer/BioNTech or Moderna mRNA vaccine revealed that they are more likely than age-matched non-pregnant women to experience injection site pain but less likely to experience headache, muscle ages, chills, and fever as side effects of the covid-19 vaccines. And, the incidence of pregnancy loss and adverse neonatal outcome (including preterm birth and small size for gestational age) were similar to incidence rates reported in studies conducted before the Covid-19 pandemic. (That’s good news and an example of v-safe in action!)
A prospective study of pregnant and lactating women showed that the mRNA vaccines induced similar levels of antibodies compared to non-pregnant and non-lactating women, and higher levels of antibodies than pregnant women after natural infection. And vaccine-generated antibodies were found in all umbilical cord blood and breastmilk samples, indicating that some immune protection was transferred to the baby. A further prospective study conducted in Israel in lactating women showed that 97% of breast milk samples contained protective levels of IgG antibodies (20.5 U/mL) against SARS-CoV-2 by 5 to 6 weeks after the first shot (about 2 to 3 weeks after the second shot). Neither of these studies reported any adverse events.
The results from the Pfizer/BioNTech phase 2/3 clinical trial in adolescents aged 12 to 15 has also shown an excellent safety profile, with similar levels of immune response as seen in adults, and similar frequency of side effects (more on side effects below).
Advantages of mRNA Vaccines: Efficacy without Adjuvants
The third major advantage to mRNA vaccines is efficacy. Our cells can actually produce much more antigenic protein via this platform than can be delivered using traditional vaccine platforms like subunit, conjugate, live attenuated or inactivated virus. The protein is then presented to the immune system via the major histocompatibility complex (MHC). Additionally, in the case of the covid-19 vaccines, the spike protein mRNA was slightly modified to add a transmembrane anchor, so the antigenic coronavirus spike protein also presents itself to our immune systems! Another advantage of adding this transmembrane anchor is that the spike protein produced by our cells after vaccination doesn’t enter the circulation (i.e., the bloodstream), so it can’t bind with ACE2 receptors and interfere with our renin-angiotensin system. (As an aside, both the Johnson & Johnson/Janssen and AstraZeneca adenovirus vector vaccines deliver DNA instructions for a modified spike protein to also include a transmembrane anchor! Yay!)
The first injection primes the immune system and the booster shot a few weeks later ensures a more robust and durable immunological memory! This elicits an effective and strong T cell and humoral immune response—without the need for adjuvants! In fact, that second shot of the mRNA vaccines increases our production of neutralizing antibodies by about 10 times! And, that doesn’t just increase our protection, it also leads to longer-lasting (i.e., more durable) immunity.
Adjuvants (most commonly aluminum-based molecules) are added to traditional vaccines to stimulate the immune system so we develop a more robust immunity against the antigen. This broad immune stimulation is why people with autoimmune disease and other inflammatory diseases can sometimes experience a flare after vaccination. However, the immune response after vaccination with mRNA vaccines is targeted against the foreign protein, activating the same pathways that our immune systems use to fight off any virus. Certainly, a transient increase in autoimmune symptoms is still possible, in the same way that fighting off a cold or flu can trigger increased symptoms, but the lack of adjuvant in these vaccines is very good news for autoimmune disease sufferers.
In fact, the ingredients of these vaccines are devoid or worrisome compounds (PEG allergy excepted). For example, the Moderna vaccine contains the following clean ingredients:
- the mRNA strand,
- four lipids which make up the lipid envelope (SM-102, PEG2000-DMG, cholesterol, and DSPC),
- four pH buffering agents (tromethamine and tromethamine hydrochloride, which are both common drugs for metabolic acidosis, and acetic acid and sodium acetate, which are both naturally found in our blood), and
- sucrose as a cryo-stabilizer.
And that’s it! No wonky preservatives, stabilizers or residuals!
The Pfizer/BioNTech and Moderna phase 2/3 clinical trials showed that both vaccines prevented about 95% of symptomatic covid-19 infections. Both vaccines were at least 90% effective at preventing severe and critical courses of covid-19 and 100% effective at preventing death from covid-19. The results from the Pfizer/BioNTech phase 2/3 clinical trial in adolescents aged 12 to 15 showed 100% efficacy against symptomatic covid-19 infection (this age group is at much lower risk of severe disease and death, so this clinical trial couldn’t test efficacy, but it’s expected to also be high). That’s highly efficacious!
Sub-group analysis of the phase 2/3 clinical trial results for both the mRNA vaccines—stratified by age, race, ethnicity, sex, baseline BMI, and the presence of known risk factors for severe disease, including chronic lung disease, significant cardiac disease, severe obesity, diabetes, liver disease and HIV infection—revealed similar vaccine efficacy (85% to 100%) across all subgroups. Autoimmune disease is not a risk factor for severe covid-19, unless you are taking immunosuppressant drugs to manage your disease. So, people with autoimmune disease were included in the covid-19 clinical trials but not analyzed separately in the sub-group analysis. In the detailed adverse event reports included in the FDA Briefing Documents for both Pfizer/BioNTech and Moderna, there is no difference between vaccine and placebo groups in terms of new autoimmune disease incidence or symptom flares of preexisting autoimmune disease. (It’s worth noting here that this is consistent with several large-scale prospective studies of traditional vaccines that indicate absolutely no link between vaccines and autoimmune disease or autoantibody formation, like this one.) More good news!
This high efficacy and safety across all subgroups is excellent news! And, even better, real-world data also bears this out!
In a CDC study, 3950 healthcare workers, first responders and frontline workers (who have high risk of workplace exposure) where tested every week during and after vaccination with either of the mRNA vaccines, capturing both asymptomatic covid-19 infections and symptomatic ones. The vaccines yielded 80% immunity by 2 weeks after the first vaccination and 90% immunity by 2 weeks after the booster.
A similarly designed study of healthcare workers in Israel who were vaccinated with the Pfizer/BioNTech covid-19 vaccine, showed a 97% reduction in symptomatic infections and an 86% reduction in asymptomatic infections in fully vaccinated healthcare workers compared to unvaccinated healthcare workers.
In a Mayo Clinic study, over 39,000 asymptomatic adult patients were screened for covid-19 prior to surgery or other procedures. The first vaccine dose prevented 72% of asymptomatic infections while full vaccination prevented 80% of asymptomatic infections.
And a study of hospitalized adults over the age of 65 in the USA who received either the Pfizer/BioNTech or Moderna covid-19 vaccines, the first shot was 64% effective at preventing a severe case of covid-19 requiring hospitalization whereas full vaccination was 94% effective at preventing severe disease requiring hospitalization.
That’s more good news, including real-world data showing vaccine efficacy against asymptomatic infections, too!
In fact, there are very low numbers of breakthrough infections after full vaccination (i.e., getting covid-19 even after being fully vaccinated). Some breakthrough infections are expected because none of the vaccines are 100% protective. The CDC reports that, as of April 26, 2021, there were only 9,245 covid-19 positive infections among the over 95,000,000 fully vaccinated Americans at that time. Also, as expected, almost all of the breakthrough infections were asymptomatic or mild. A very, very small number (about 6.4%) of breakthrough infections were severe enough to require hospitalization, including 122 older adults with preexisting conditions who died from covid-19 after being vaccinated. Studies have also shown that viral load is about 4-fold lower in fully vaccinated people with breakthrough infections, so while you can still be contagious if you get a breakthrough infection, you are much less so than unvaccinated people. Even more good news, the covid-19 cases are dropping as a result of vaccinations (and probably some other helpful factors like warm summer weather), so hopefully we’ll get to a point where vaccination means you can throw those masks away soon! Hang in there! See also Covid-19 FAQ: Do Face Masks Even Work?
Okay, time for an important aside: This article is focused on the covid-19 mRNA vaccines, but the two adenovirus vector DNA vaccines: Johnson & Johnson/Janssen and AstraZeneca/Oxford vaccines also do not include adjuvants and have very clean ingredients. The concept behind these vaccines is similar to the mRNA vaccines in the sense that they deliver the instructions for our cells to produce the spike protein of SARS-CoV-2. In this case, an adenovirus (which normally causes a mild or asymptomatic common cold and completely lacks the enzymes require to alter our DNA, making them an excellent DNA delivery vehicle for vaccines) that has been made replication incompetent delivers the DNA instructions to our cell nuclei, which then transcribe the DNA into mRNA and then translate the mRNA into membrane-bound coronavirus spike protein. These vaccines are also built on decades of research (I actually used adenoviruses for gene therapy research during my PhD!). Their efficacy profiles are similar (about 70% effective against symptomatic infection, 80% effective against severe and critical infection, and 100% effective at preventing death from covid-19), but do note that we can’t directly compare these to the mRNA vaccines since they were tested at different times in different countries after variants of concern became dominant). And, both adenovirus vector vaccines have a similar very low frequency occurrence of one notable adverse event, immune thrombotic thrombocytopenia (misrepresented in the media as “blood clots”). I have created a more in-depth resource where I go into detail about these vaccines here: TWV Podcast Episode 454: J&J and AstraZeneca Covid-19 Vaccines.
More Durable Immunity than Natural Infection
Early data shows a longer-lasting production of neutralizing antibodies against the SARS-CoV-2 spike protein after vaccination than natural infection, with higher levels of neutralizing antibodies four months after vaccination compared to four months after natural infection.
Immunity arising from natural infection with covid-19 is likely limited—while the absolute numbers of confirmed covid-19 reinfections remain a fraction of a percent, studies in other coronaviruses imply that natural immunity does wane. Immunity from common cold-causing coronaviruses lasts about a year, and immunity from SARS-CoV (responsible for the 2002-2003 SARS pandemic, also the coronavirus most similar to SARS-CoV-2, about 80% homology) lasted up to six years (see TWV Podcast Episode 425: Covid-19 FAQ Part 4). This is likely attributable to the fact that the novel coronavirus manipulates our immunes systems to evade detection, downregulating interferon production and interfering with antigen presentation by the MHC—this manipulation of our immune response is absent in the covid-10 vaccines (see Covid-19 FAQ: Do Face Masks Even Work?).
This is why public health officials recommend getting vaccinated even if you’ve already had covid-19, waiting until you have completely recovered from infection or 90 days, whichever is less.
This is also why it’s so important not to skip your second vaccine dose! As I already mentioned, neutralizing antibodies go up about 10 times after the booster shot, which increases efficacy and is also expected to notably increase the durability of immunity, i.e., how long of a period of time the vaccines protect us. The booster also dramatically increases protection against the variants of concern. For example, newly published data from Israel where the B.1.1.7 and B.1.351 variants dominate show only about 58% protection after one dose of the Pfizer/BioNTech vaccine (76% protection against hospitalization and 77% protection against death), but this increases to 96% protection against infection (98% protection against hospitalization and death) by two weeks after the second shot. So far, even the participants of the earliest vaccine studies still have protective levels of antibodies in their blood. They will be monitored for at least two years, and the persistence of antibodies will help determine whether or not regular boosters are required and on what timescale (anywhere from annual to every 5 years is likely based on what we know about immunity to other coronaviruses like those causing the common cold, SARS and MERS).
Speaking of making sure to get your second dose… If you received your first shot, but missed your window for the second shot (3-6 weeks for Pfizer/BioNTech and 4-6 weeks for Moderna) for whatever reason, emerging evidence says it’s still worthwhile to go ahead and get your booster at your earliest opportunity. (No, you don’t need to restart your vaccine course.) A brand-new unpublished study evaluating a 3-week timing between the two Pfizer/BioNTech inoculations versus a 12-week timing in adults over the age of 80 actually showed higher antibody levels with the longer timing! This doesn’t mean we should all delay (this research still needs to be peer0reviewed and it was performed in a specific demographic), but it does point to it being okay if life got in the way of that second dose and you’re now finding yourself in the situation where you need to catch up.
Pfizer vs. Moderna (vs. J&J)
There is very little difference between the Pfizer/BioNTech and Moderna covid-19 vaccines. Moderna has spent about ten years perfecting their lipid nanoparticle technology, which is why their vaccine is more stable and doesn’t require ultra-cold temperatures for storage and shipping. The mRNA sequences they deliver are nearly identical, and the differences in safety and efficacy between the two vaccines are within normal variability and standard deviation, meaning they’re statistically equivalent. While it hypothetically shouldn’t matter if you mixed and matched these two vaccines, because it hasn’t been specifically tested, best practice is to get both initial injection and booster from the same vaccine manufacturer.
It’s also helpful to note here that we can’t compare efficacy of the mRNA vaccines to the Johnson & Johnson/Janssen or AstraZeneca/Oxford vaccine, because they were tested at different times and in different countries, when different variants were dominant. To actually be able to make a statement of one vaccine being more efficacious than another, we’d need a head-to-head comparison trial. This is discussed more in TWV Podcast Episode 454: J&J and AstraZeneca Covid-19 Vaccines.
Post Inoculation Side Effects – What to Expect
The most common side effect of the mRNA vaccines are injection site reactions (like arm pain or bruising), which impacts about 90% of people. Systemic side effects—any or a combination of fatigue, headache, muscle aches, chills, joint pain, fever, nausea, vomiting and diarrhea—affect about half of people receiving the vaccine and are short-lived (up to a couple of days) and similar in frequency and severity to other traditional vaccines. It is common for side effects to be a little worse after the booster shot compared to the first injection, but there are plenty of counterexamples to point to. Some women also experience menstrual irregularities following the vaccination, likely mediated via cortisol (which increases during immune activation and infection and which impacts the hypothalamic-pituitary-gonadal axis, discussed in detail in TWV Podcast Episode 455: Covid-19 Vaccines – Real World Data and Updated Vaccine Studies and see How Chronic Stress Leads to Hormone Imbalance).
I can tell you that I had some mild side effects from the first shot that lasted about 36 hours (low-grade fever, mild chills, arm pain, and mild fatigue), so I planned ahead for the second shot and filled my fridge with leftovers and cleared my schedule. I definitely had more intense side effects after the second shot, about 30 hours of a sore arm and bad flu-like symptoms (fever, chills, muscle aches, joint pain, headache, stomach upset and fatigue), followed by two or three days of lingering mild joint pain. A great trade that I would do again in a heartbeat!
It’s important to differentiate here between a side effect and an adverse reaction. A side effect is an unpleasant but overall harmless secondary effect, whereas an adverse reaction is an unwanted/unexpected and dangerous reaction to a therapeutic. These side effects are all signs that the immune system is doing its job! Also, severity of side effects does not correlate with immunity, so having no side effect at all does not mean that you aren’t protected.
The same ways you would support your immune system if recovering from illness can help if you experience side effects on the more unpleasant end of the spectrum: rest, hydration and nutrient-density.
Busting Covid-19 Vaccine Myths
The many pervasive myths circulating on the internet about these covid-19 vaccines are simply false. No corners were cut in the testing of these vaccines; just like any new drug, community monitoring is important, but we are not guinea pigs here. Emergency Use Authorization does not mean that the vaccines weren’t fully and rigorously tested; it is an expedited approval system designed exactly for emergency situations like global pandemics. Pfizer/BioNTech has already applied for full FDA approval (the other vaccine manufacturers won’t be far behind), the main difference is longer duration of observation of the clinical trial participants (6 months worth of data compared to the 3ish months worth when they were first applied for their EUA).
The vaccines do not contain whole, intact virus and can not give you covid-19. The vaccines do not make you contagious—people can’t “catch the vaccine” from you. No immortalized cell lines originally derived from fetal tissues were used at any stage of design, development or production of either mRNA vaccine; and none of the vaccines contain any aborted fetal cells or tissues.
There is currently no evidence of female infertility as a consequence of either natural infection nor any covid-19 vaccine—the sequence homology to placental protein syncytin-1 is so low that autoantibody formation is extremely, extremely unlikely (only 4 amino acids are homologous, but at least 10 are usually required for cross-reactivity).
There is no evidence to support the myths that the vaccine will cause autoimmune disease or antibody-enhanced infection. There is no evidence that the vaccines will negatively impact hematopoietic stem cells. The spike protein our bodies make in response to the vaccines are membrane bound so they can not bind with our ACE2 receptors or damage the cardiovascular system.
None of the vaccines alter our DNA. The vaccines do not contain tracking microchips; in fact, Faraday’s law makes that technology physically (as in the laws of physics) impossible.
I bust these myths in detail and answer FAQ in these two podcast episodes:
- TWV Podcast Episode 443: Covid-19 Vaccines Part 3 – FAQs
- TWV Podcast Episode 444: Covid-19 Vaccines Part 4 – Myth Busing
Benefits to Long Covid
Studies suggest that anywhere from 10% to 30% of people infected with covid-19 have persistent symptoms beyond 3 months, qualifying as “long covid”. Long covid can be triggered even by mild covid-19 infections. There are a couple of different possible explanations for long covid. It could be chronic fatigue syndrome, an autoimmune disease well known to have infectious triggers. Or, it could be persistent infection, meaning the immune system doesn’t quite fully vanquish the pathogen. Or some people could have chronic fatigue syndrome and others a chronic infection, or both. In any case, a vast array of symptoms are possible—including chronic cough, chest pain, shortness of breath, fatigue, brain fog, memory and sleep problems—ranging from mild to severe, and can either be consistent day-to-day or fluctuate in both symptom severity and even what symptom(s) is being experienced.
There’s emerging evidence that getting vaccinated can improve or resolve long covid, although definitely more studies are needed. While various surveys have shown that about 40% of respondents saw improvement in their long covid symptoms after getting vaccinated, a preprint study performed in the United Kingdom showed that almost a quarter of long covid patients saw symptom resolution after either the Pfizer/BioNTech or AstraZeneca/Oxford vaccine. Either way, that’s good news for long covid sufferers.
What Can You Do After Getting Vaccinated?
You are considered fully immunized two weeks after your second shot, at which point, you are at much, much, much lower risk of contracting covid-19 and passing it on to friends and family. And that means we can participate in most the activities we enjoyed pre-pandemic, mask-free and without social distancing!
New CDC guidelines for fully vaccinated people include participating many indoor activities without wearing a mask or staying 6 feet apart, including grocery shopping if the store isn’t crowded, shopping at uncrowded shopping centers, visit an uncrowded museum, visiting a barber or hair salon, go to a movie theatre, attend a full-capacity worship service, eat at an indoor restaurant or bar, and even participate in an indoor, high intensity exercise class! It’s even low-risk to participate in small indoor gatherings with vaccinated and unvaccinated people, providing none of the unvaccinated are at increased risk for severe covid-19. We can also enjoy the outdoors without a mask, even in crowded venues like stadiums and concerts. And, for everyone tired of feeling like their brains are being impaled by a nasal swab, being fully vaccinated means no more covid-19 testing when you travel or if you’ve been exposed to someone who is infected, unless you have symptoms. You also get to skip the self-quarantine when traveling domestically in the USA or if you’ve been exposed to someone who is infected (again, unless you have symptoms). And, if your employer requires regular covid-19 testing, that should also discontinue once you’re fully vaccinated.
Some places where you still need to wear a mask include medical buildings (like hospitals, long-term care homes, doctor’s offices, and urgent care centers), public transportation (like airplanes, buses, and trains), and congregate living facilities as well as any location—including local businesses and workplaces—where mask wearing is required by federal, state, local, tribal, or territorial laws, rules, regulations, or ordinances. Many retailers will continue to require masks in order to enter and shop in their stores, at least until the vaccination rates are higher. Note that if you are immunocompromised or take a medication that suppresses the your immune system, you may not be fully protected even if you are fully vaccinated, so talk to your doctor. You may opt to continue mask wearing and social distancing. You can take a covid-19 antibody test to see what your titers are and make a data-driven decision.
While asymptomatic breakthrough infections are unlikely to be contagious, symptomatic ones certainly can be. If you develop any symptoms of illness after being fully vaccinated, even mild ones, wear a mask and social distance until you can get tested for covid-19, and continue until you get your test results back or until you’re fully recovered.
What about vaccinated parents of unvaccinated kids? Unvaccinated and partially-vaccinated people still need to take precautions, but small gatherings are still considered low-risk. As a parent of one partially- and one unvaccinated kid, I’m still wearing a mask in grocery stores and other indoor locations with strangers. The good news is that, as a higher and higher percentage of the population gets vaccinated, and the daily case counts continue to decrease, more and more activities will be considered low-risk. Check the CDC guidelines webpage for updates and new guidance on low-risk mask-free activities!
Who Should Get Vaccinated?
The covid-19 vaccines have proven to be safe and effective, and they are currently our best tool in fighting this global pandemic. In the USA, the Moderna and J&J covid-19 vaccines are currently approved for everyone 18 years old and older; and the Pfizer/BioNTech covid-19 vaccine is approved for everyone 12 years old and older. A little over 35% of Americans are already fully vaccinated, but current mathematical models predict that somewhere between 70% and 90% of the population needs to be immune to SARS-CoV-2 in order for us to reach herd immunity. Herd immunity means that enough of the population is immune that the virus can no longer effectively spread from person to person. There’s even a possibility that covid-19 could be eradicated, like small pox, if enough people were to get vaccinated.
Of course, it’s important to build immune health with informed diet and lifestyle and by nurturing the gut microbiome, but there is no “natural” supplement or nutrient or diet or workout or amount of sleep that can make us immune to covid-19 (see Natural Approaches to Cold & Flu Season (and Covid-19!), Covid-19 and the Gut, TPV Podcast Episode 396: Covid-19 FAQ and TWV Podcast Episode 412: Covid-19 FAQ, Part 3). Our only pathway to immunity (other than surviving a covid-19 infection, which as we’ve already discussed, includes high risk of morbidity and mortality) is getting the vaccine.
So, who should get vaccinated? Everyone who doesn’t have a contraindication diagnosed by their doctor. Yes, the vaccines do provide personal protection against covid-19, but the bigger reason for getting vaccinated is to protect our communities. While age and preexisting conditions certainly increase risk of morbidity and mortality from covid-19, there are plenty of tragic stories of perfectly healthy young people succumbing to covid-19. Herd immunity means we no longer have to roll the dice and hope that if we get it, we’ll get a mild or asymptomatic case and not suffer from long covid. And, in a way, we are in a race against the SARS-CoV-2 virus. Every new person infected is an opportunity for the virus to mutate, and it is possible for a new variant of concern to emerge that the vaccines do not confer protection against, resetting the pandemic clock and putting us all back to those dark days of Spring 2020. Even if you deem yourself to be at low risk, you could pass the virus on to somebody who is more vulnerable to severe infection or dying from covid-19, or you could be ground zero for a new variant of concern that the vaccine doesn’t protect against. If you’re unsure about whether the vaccine is safe for you given your personal health history, talk to your doctor.
I completely understand and have compassion for vaccine hesitancy, so I earnestly hope that his article has answered your questions, assuaged your fears, and helped you feel empowered with facts and information. And, as a reminder, if you still have follow-up questions, I go into even more detail on the science behind the covid-19 vaccines in:
- TWV Podcast Episode 440: Covid-19 Vaccines Part 1 – mRNA Vaccine Technology
- TWV Podcast Episode 441: Covid-19 Vaccines Part 2 – Pfizer/BioNTech vs Moderna
- TWV Podcast Episode 443: Covid-19 Vaccines Part 3 – FAQs
- TWV Podcast Episode 444: Covid-19 Vaccines Part 4 – Myth Busing
- TWV Podcast Episode 454: J&J and AstraZeneca Covid-19 Vaccines
- TWV Podcast Episode 455: Covid-19 Vaccines – Real World Data and Updated Studies
Citations
Abu-Raddad LJ, Chemaitelly H, Butt AA; National Study Group for COVID-19 Vaccination. Effectiveness of the BNT162b2 Covid-19 Vaccine against the B.1.1.7 and B.1.351 Variants. N Engl J Med. 2021 May 5. doi: 10.1056/NEJMc2104974.
Angel Y, Spitzer A, Henig O, Saiag E, Sprecher E, Padova H, Ben-Ami R. Association Between Vaccination With BNT162b2 and Incidence of Symptomatic and Asymptomatic SARS-CoV-2 Infections Among Health Care Workers. JAMA. 2021 May 6. doi: 10.1001/jama.2021.7152. \
Arnold DT, Milne A, Samms E, Stadon L, Maskell NA, Hamilton FW. Are vaccines safe in patients with Long COVID? A prospective observational study. medRxiv 2021.03.11.21253225; doi: 10.1101/2021.03.11.21253225.
Baden LR, El Sahly HM, Essink B, Kotloff K, Frey S, Novak R, Diemert D, Spector SA, Rouphael N, Creech CB, McGettigan J, Khetan S, Segall N, Solis J, Brosz A, Fierro C, Schwartz H, Neuzil K, Corey L, Gilbert P, Janes H, Follmann D, Marovich M, Mascola J, Polakowski L, Ledgerwood J, Graham BS, Bennett H, Pajon R, Knightly C, Leav B, Deng W, Zhou H, Han S, Ivarsson M, Miller J, Zaks T; COVE Study Group. Efficacy and Safety of the mRNA-1273 SARS-CoV-2 Vaccine. N Engl J Med. 2021 Feb 4;384(5):403-416. doi: 10.1056/NEJMoa2035389.
Dan JM, Mateus J, Kato Y, Hastie KM, Yu ED, Faliti CE, Grifoni A, Ramirez SI, Haupt S, Frazier A, Nakao C, Rayaprolu V, Rawlings SA, Peters B, Krammer F, Simon V, Saphire EO, Smith DM, Weiskopf D, Sette A, Crotty S. Immunological memory to SARS-CoV-2 assessed for up to 8 months after infection. Science. 2021 Feb 5;371(6529):eabf4063. doi: 10.1126/science.abf4063.
Ferlito C, Barnaba V, Abrignani S, Bombaci M, Sette A, Sidney J, Biselli R, Tomao E, Cattaruzza MS, Germano V, Biondo MI, Salerno G, Lulli P, Caporuscio S, Picchianti Diamanti A, Falco M, Biselli V, Cardelli Ferlito C, Barnaba V, Abrignani S, Bombaci M, Sette A, Sidney J, Biselli R, Tomao E, Cattaruzza MS, Germano V, Biondo MI, Salerno G, Lulli P, Caporuscio S, Picchianti Diamanti A, Falco M, Biselli V, Cardelli P, Autore A, Lucertini E, De Cesare DP, Peragallo MS, Lista F, Martire C, Salemi S, Nisini R, D’Amelio R. Lack of evidence for post-vaccine onset of autoimmune/lymphoproliferative disorders, during a nine-month follow-up in multiply vaccinated Italian military personnel. Clin Immunol. 2017 Aug;181:60-66. doi: 10.1016/j.clim.2017.06.001.
Gray KJ, Bordt EA, Atyeo C, Deriso E, Akinwunmi B, Young N, Medina Baez A, Shook LL, Cvrk D, James K, De Guzman R, Brigida S, Diouf K, Goldfarb I, Bebell LM, Yonker LM, Fasano A, Rabi SA, Elovitz MA, Alter G, Edlow AG. COVID-19 vaccine response in pregnant and lactating women: a cohort study. Am J Obstet Gynecol. 2021 Mar 24:S0002-9378(21)00187-3. doi: 10.1016/j.ajog.2021.03.023.
Hansen CH, Michlmayr D, Gubbels SM, Mølbak K, Ethelberg S. Assessment of protection against reinfection with SARS-CoV-2 among 4 million PCR-tested individuals in Denmark in 2020: a population-level observational study. Lancet. 2021 Mar 27;397(10280):1204-1212. doi: 10.1016/S0140-6736(21)00575-4.
Jackson LA, Anderson EJ, Rouphael NG, Roberts PC, Makhene M, Coler RN, McCullough MP, Chappell JD, Denison MR, Stevens LJ, Pruijssers AJ, McDermott A, Flach B, Doria-Rose NA, Corbett KS, Morabito KM, O’Dell S, Schmidt SD, Swanson PA 2nd, Padilla M, Mascola JR, Neuzil KM, Bennett H, Sun W, Peters E, Makowski M, Albert J, Cross K, Buchanan W, Pikaart-Tautges R, Ledgerwood JE, Graham BS, Beigel JH; mRNA-1273 Study Group. An mRNA Vaccine against SARS-CoV-2 – Preliminary Report. N Engl J Med. 2020 Nov 12;383(20):1920-1931. doi: 10.1056/NEJMoa2022483.
Jackson NAC, Kester KE, Casimiro D, Gurunathan S, DeRosa F. The promise of mRNA vaccines: a biotech and industrial perspective. NPJ Vaccines. 2020 Feb 4;5:11. doi: 10.1038/s41541-020-0159-8.
Lee WS, Wheatley AK, Kent SJ, DeKosky BJ. Antibody-dependent enhancement and SARS-CoV-2 vaccines and therapies. Nat Microbiol. 2020 Oct;5(10):1185-1191. doi: 10.1038/s41564-020-00789-5. Epub 2020 Sep 9.
Levine-Tiefenbrun M, Yelin I, Katz R, Herzel E, Golan Z, Schreiber L, Wolf T, Nadler V, Ben-Tov A, Kuint J, Gazit S, Patalon T, Chodick G, Kishony R. Initial report of decreased SARS-CoV-2 viral load after inoculation with the BNT162b2 vaccine. Nat Med. 2021 Mar 29. doi: 10.1038/s41591-021-01316-7.
Li YD, Chi WY, Su JH, Ferrall L, Hung CF, Wu TC. Coronavirus vaccine development: from SARS and MERS to COVID-19. J Biomed Sci. 2020 Dec 20;27(1):104. doi: 10.1186/s12929-020-00695-2.
Logue JK, Franko NM, McCulloch DJ, McDonald D, Magedson A, Wolf CR, Chu Hy. Sequelae in Adults at 6 Months After COVID-19 Infection. JAMA Netw Open. 2021;4(2):e210830. doi:10.1001/jamanetworkopen.2021.0830
Pardi N, Hogan MJ, Porter FW, Weissman D. mRNA vaccines – a new era in vaccinology. Nat Rev Drug Discov. 2018 Apr;17(4):261-279. doi: 10.1038/nrd.2017.243.
Pascutti MF, Erkelens MN, Nolte MA. Impact of Viral Infections on Hematopoiesis: From Beneficial to Detrimental Effects on Bone Marrow Output. Front Immunol. 2016 Sep 16;7:364. doi: 10.3389/fimmu.2016.00364.
Perl SH, Uzan-Yulzari A, Klainer H, Asiskovich L, Youngster M, Rinott E, Youngster I. SARS-CoV-2-Specific Antibodies in Breast Milk After COVID-19 Vaccination of Breastfeeding Women. JAMA. 2021 Apr 12:e215782. doi: 10.1001/jama.2021.5782.
Petter E, Mor O, Zuckerman N, Oz-Levi D, Younger A, Aran D, Erlich Y. Initial real world evidence for lower viral load of individuals who have been vaccinated by BNT162b2. medRxiv 2021.02.08.21251329; doi: 10.1101/2021.02.08.21251329.
Polack FP, Thomas SJ, Kitchin N, Absalon J, Gurtman A, Lockhart S, Perez JL, Pérez Marc G, Moreira ED, Zerbini C, Bailey R, Swanson KA, Roychoudhury S, Koury K, Li P, Kalina WV, Cooper D, Frenck RW Jr, Hammitt LL, Türeci Ö, Nell H, Schaefer A, Ünal S, Tresnan DB, Mather S, Dormitzer PR, Şahin U, Jansen KU, Gruber WC; C4591001 Clinical Trial Group. Safety and Efficacy of the BNT162b2 mRNA Covid-19 Vaccine. N Engl J Med. 2020 Dec 31;383(27):2603-2615. doi: 10.1056/NEJMoa2034577.
Poland GA, Ovsyannikova IG, Kennedy RB. SARS-CoV-2 immunity: review and applications to phase 3 vaccine candidates. Lancet. 2020 Nov 14;396(10262):1595-1606. doi: 10.1016/S0140-6736(20)32137-1.
Reichmuth AM, Oberli MA, Jaklenec A, Langer R, Blankschtein D. mRNA vaccine delivery using lipid nanoparticles. Ther Deliv. 2016;7(5):319-34. doi: 10.4155/tde-2016-0006. Erratum in: Ther Deliv. 2016 Jun;7(6):411.
Sahin U, Karikó K, Türeci Ö. mRNA-based therapeutics–developing a new class of drugs. Nat Rev Drug Discov. 2014 Oct;13(10):759-80. doi: 10.1038/nrd4278.
Schattner A. Consequence or coincidence? The occurrence, pathogenesis and significance of autoimmune manifestations after viral vaccines. Vaccine. 2005 Jun 10;23(30):3876-86. doi: 10.1016/j.vaccine.2005.03.005. Epub 2005 Apr 7.
Shimabukuro TT, Kim SY, Myers TR, Moro PL, Oduyebo T, Panagiotakopoulos L, Marquez PL, Olson CK, Liu R, Chang KT, Ellington SR, Burkel VK, Smoots AN, Green CJ, Licata C, Zhang BC, Alimchandani M, Mba-Jonas A, Martin SW, Gee JM, Meaney-Delman DM; CDC v-safe COVID-19 Pregnancy Registry Team. Preliminary Findings of mRNA Covid-19 Vaccine Safety in Pregnant Persons. N Engl J Med. 2021 Apr 21. doi: 10.1056/NEJMoa2104983.
Spencer JP, Trondsen Pawlowski RH, Thomas S. Vaccine Adverse Events: Separating Myth from Reality. Am Fam Physician. 2017 Jun 15;95(12):786-794.
Tenforde MW, Olson SM, Self WH, Talbot HK, Lindsell CJ, Steingrub JS, Shapiro NI, Ginde AA, Douin DJ, Prekker ME, Brown SM, Peltan ID, Gong MN, Mohamed A, Khan A, Exline MC, Files DC, Gibbs KW, Stubblefield WB, Casey JD, Rice TW, Grijalva CG, Hager DN, Shehu A, Qadir N, Chang SY, Wilson JG, Gaglani M, Murthy K, Calhoun N, Monto AS, Martin ET, Malani A, Zimmerman RK, Silveira FP, Middleton DB, Zhu Y, Wyatt D, Stephenson M, Baughman A, Womack KN, Hart KW, Kobayashi M, Verani JR, Patel MM; IVY Network; HAIVEN Investigators. Effectiveness of Pfizer-BioNTech and Moderna Vaccines Against COVID-19 Among Hospitalized Adults Aged ≥65 Years – United States, January-March 2021. MMWR Morb Mortal Wkly Rep. 2021 May 7;70(18):674-679. doi: 10.15585/mmwr.mm7018e1.
Thompson MG, Burgess JL, Naleway AL, Tyner HL, Yoon SK, Meece J, Olsho LEW, Caban-Martinez AJ, Fowlkes A, Lutrick K, Kuntz JL, Dunnigan K, Odean MJ, Hegmann KT, Stefanski E, Edwards LJ, Schaefer-Solle N, Grant L, Ellingson K, Groom HC, Zunie T, Thiese MS, Ivacic L, Wesley MG, Lamberte JM, Sun X, Smith ME, Phillips AL, Groover KD, Yoo YM, Gerald J, Brown RT, Herring MK, Joseph G, Beitel S, Morrill TC, Mak J, Rivers P, Harris KM, Hunt DR, Arvay ML, Kutty P, Fry AM, Gaglani M. Interim Estimates of Vaccine Effectiveness of BNT162b2 and mRNA-1273 COVID-19 Vaccines in Preventing SARS-CoV-2 Infection Among Health Care Personnel, First Responders, and Other Essential and Frontline Workers – Eight U.S. Locations, December 2020-March 2021. MMWR Morb Mortal Wkly Rep. 2021 Apr 2;70(13):495-500. doi: 10.15585/mmwr.mm7013e3.
Walsh EE, Frenck RW Jr, Falsey AR, Kitchin N, Absalon J, Gurtman A, Lockhart S, Neuzil K, Mulligan MJ, Bailey R, Swanson KA, Li P, Koury K, Kalina W, Cooper D, Fontes-Garfias C, Shi PY, Türeci Ö, Tompkins KR, Lyke KE, Raabe V, Dormitzer PR, Jansen KU, Şahin U, Gruber WC. Safety and Immunogenicity of Two RNA-Based Covid-19 Vaccine Candidates. N Engl J Med. 2020 Dec 17;383(25):2439-2450. doi: 10.1056/NEJMoa2027906.
Widge AT, Rouphael NG, Jackson LA, Anderson EJ, Roberts PC, Makhene M, Chappell JD, Denison MR, Stevens LJ, Pruijssers AJ, McDermott AB, Flach B, Lin BC, Doria-Rose NA, O’Dell S, Schmidt SD, Neuzil KM, Bennett H, Leav B, Makowski M, Albert J, Cross K, Edara VV, Floyd K, Suthar MS, Buchanan W, Luke CJ, Ledgerwood JE, Mascola JR, Graham BS, Beigel JH; mRNA-1273 Study Group. Durability of Responses after SARS-CoV-2 mRNA-1273 Vaccination. N Engl J Med. 2021 Jan 7;384(1):80-82. doi: 10.1056/NEJMc2032195. Epub 2020 Dec 3. PMID: 33270381; PMCID: PMC7727324.
Zhang C, Maruggi G, Shan H, Li J. Advances in mRNA Vaccines for Infectious Diseases. Front Immunol. 2019 Mar 27;10:594. doi: 10.3389/fimmu.2019.00594.















 TWV Podcast Episode 455: Covid-19 Vaccines – Real World Data and Updated Vaccine Studies
TWV Podcast Episode 455: Covid-19 Vaccines – Real World Data and Updated Vaccine Studies