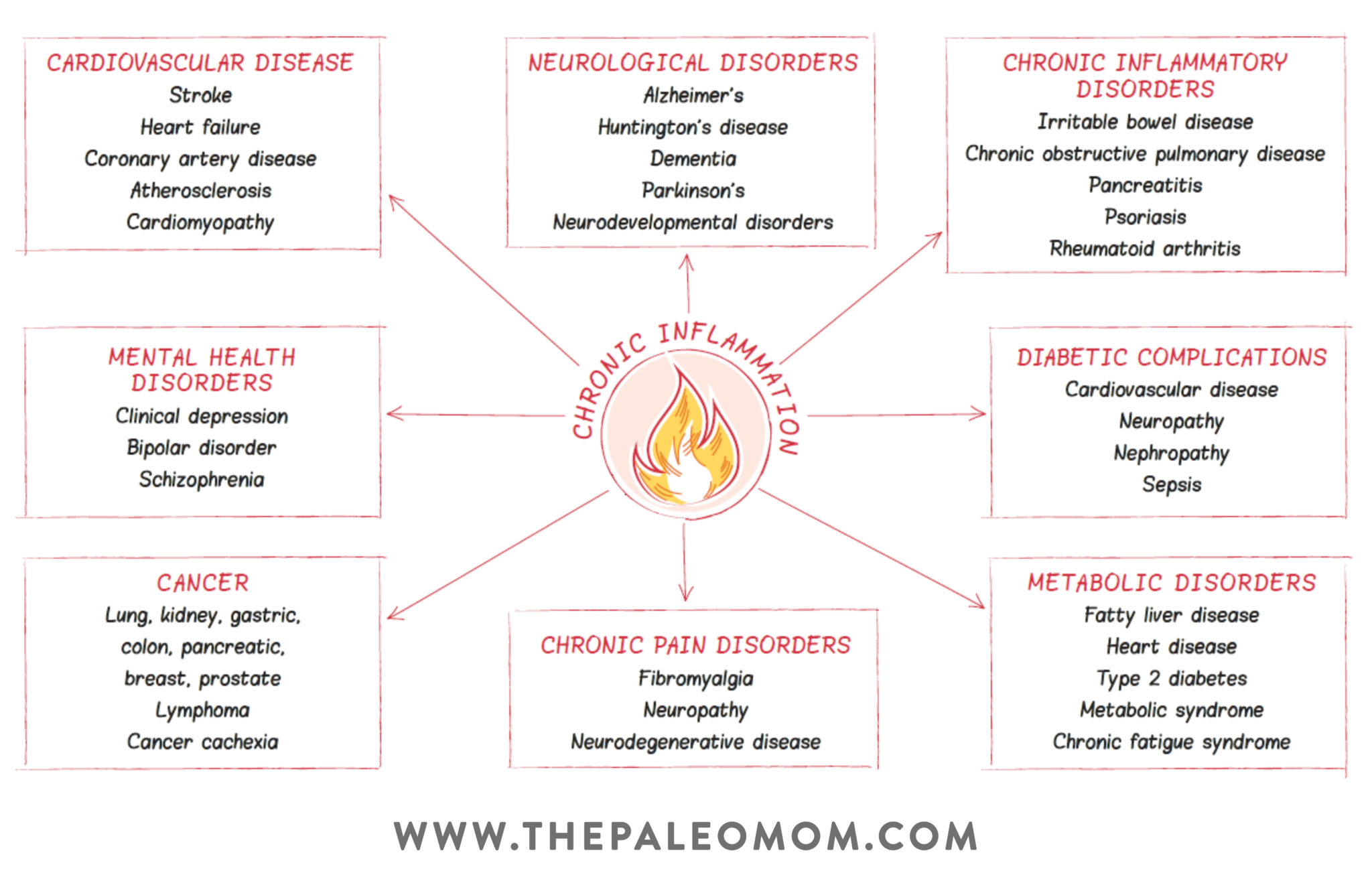
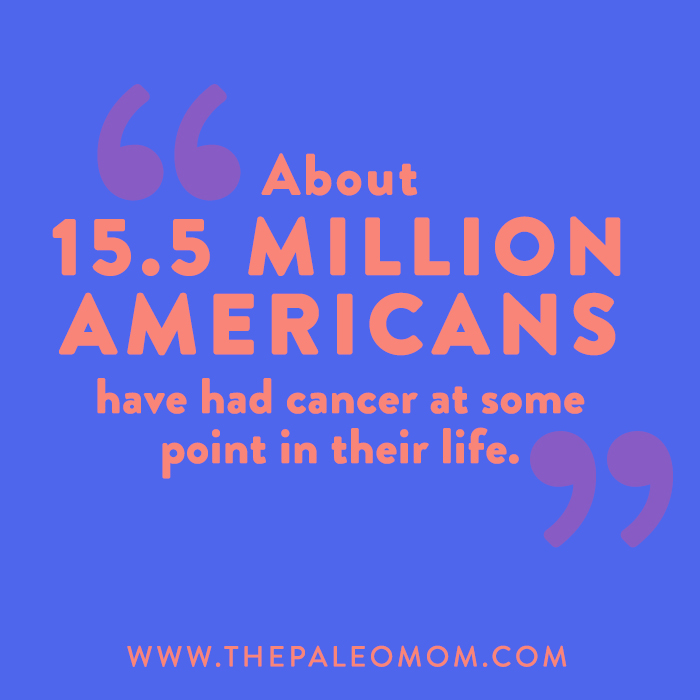
According to our best estimates, about 15.5 million Americans have had cancer at some point in their life, and about as many as 60 million Americans have an autoimmune disease. That means nearly all of us will eventually be impacted by these conditions—whether we ourselves get a diagnosis, or someone close to us does. And, that makes it extra important to understand the intricate (and under-discussed!) relationship between cancer and autoimmune disease. That’s right: along with being unfortunately common, these two forms of disease have direct biological links with each other!
Table of Contents[Hide][Show]
 In a nutshell, autoimmune disease and cancer both arise from dysfunctions of the immune system—but those dysfunctions are extremely different! Autoimmunity occurs when the body mistakenly attacks itself, launching an immune response against self-antigens that aren’t actually harmful. Cancer occurs when the body’s mechanisms for controlling cell growth and death malfunction, preventing old cells from dying and allowing them to proliferate out of control. Despite the dramatic differences in their underlying mechanisms, for many years, researchers have observed that certain autoimmune diseases are positively or negatively associated with certain cancers. For example, Crohn’s disease comes with a higher risk of several gastrointestinal cancers, while multiple sclerosis appears to reduce the risk of gastrointestinal cancers. These types of links are repeated across multiple studies, are often highly statistically significant, and involve a huge range of both autoimmune diseases and cancer types. What’s going on here?!
In a nutshell, autoimmune disease and cancer both arise from dysfunctions of the immune system—but those dysfunctions are extremely different! Autoimmunity occurs when the body mistakenly attacks itself, launching an immune response against self-antigens that aren’t actually harmful. Cancer occurs when the body’s mechanisms for controlling cell growth and death malfunction, preventing old cells from dying and allowing them to proliferate out of control. Despite the dramatic differences in their underlying mechanisms, for many years, researchers have observed that certain autoimmune diseases are positively or negatively associated with certain cancers. For example, Crohn’s disease comes with a higher risk of several gastrointestinal cancers, while multiple sclerosis appears to reduce the risk of gastrointestinal cancers. These types of links are repeated across multiple studies, are often highly statistically significant, and involve a huge range of both autoimmune diseases and cancer types. What’s going on here?!
The Link Between Cancer and Autoimmune Disease: Th1 vs. Th2 Balance
In order to understand how cancer and autoimmunity are linked, we first need to understand their relationship with the immune system!
While we sleep, eat, sit in traffic, check Facebook, and perform the hundreds of other tasks that make up our day-to-day life, our immune system is hard at work protecting us from both outside invaders and internal malfunctions. Those complex processes are often grouped into one of two main branches: innate immunity (our first line of defense against invaders, which includes nonspecific protective mechanisms like the skin, gut mucosa, and macrophages in the blood) and adaptive immunity (antigen-specific immune responses, where the body creates antibodies to bind the unique antigens on a particular pathogen).

When it comes to cancer and autoimmune disease, we’re mostly concerned with adaptive immunity.
In adaptive immunity, lymphocytes called T-helper cells (or Helper T cells, or Th for short) play a hugely central role. T-helper cells spot foreign pathogens, activate B cells (which secrete antibodies), activate macrophages (which eat and destroy invaders), and activate cytotoxic T cells (which kill infected target cells)… basically, they mobilize our body’s specialized army! And, there are two specific subsets of T-helper cells that are particularly relevant, defined by their cytokine (hormonal messenger protein) secretion patterns, Th1 and Th2.
In general, Th1 cells produce cytokines that cause proinflammatory responses, with the goal of destroying harmful invaders. The main Th1 cytokine is interferon-gamma (critical for immunity against viral and some bacterial infections), but Th1 cells also produce tumor necrosis factor (TNF) alpha/beta and interleukin-2. Th1 cells are involved with what’s called the “cellular immunity” pathway, which focuses on fighting viruses and other pathogens that can get inside our cells. Th1 responses also help eliminate cancerous cells, and when an attack is mis-directed towards our own tissue, Th1 cells can become heavily involved in autoimmunity.
Meanwhile, Th2 cells produce interleukins 4, 5, and 13, which promote IgE and eosinophilic responses (involved in allergic reactions). Th2 cells also produce interleukin-10, which has anti-inflammatory activity. The Th2 cells are involved in the “humoral immunity” pathway, which focuses on creating antibodies to bind pathogens outside of our cells (such as pathogens found in blood and other body fluids).
So, what does this have to do with autoimmunity or cancer? Under normal circumstances, Th1 and Th2 responses help keep each other in check. Depending on the specific immune challenge at hand, our body should be able to increase or decrease the activity of each pathway and prevent either one from chronically dominating (which is important, because T1 cells can suppress the activity of T2 cells, and vice versa, so they both need careful regulation!).
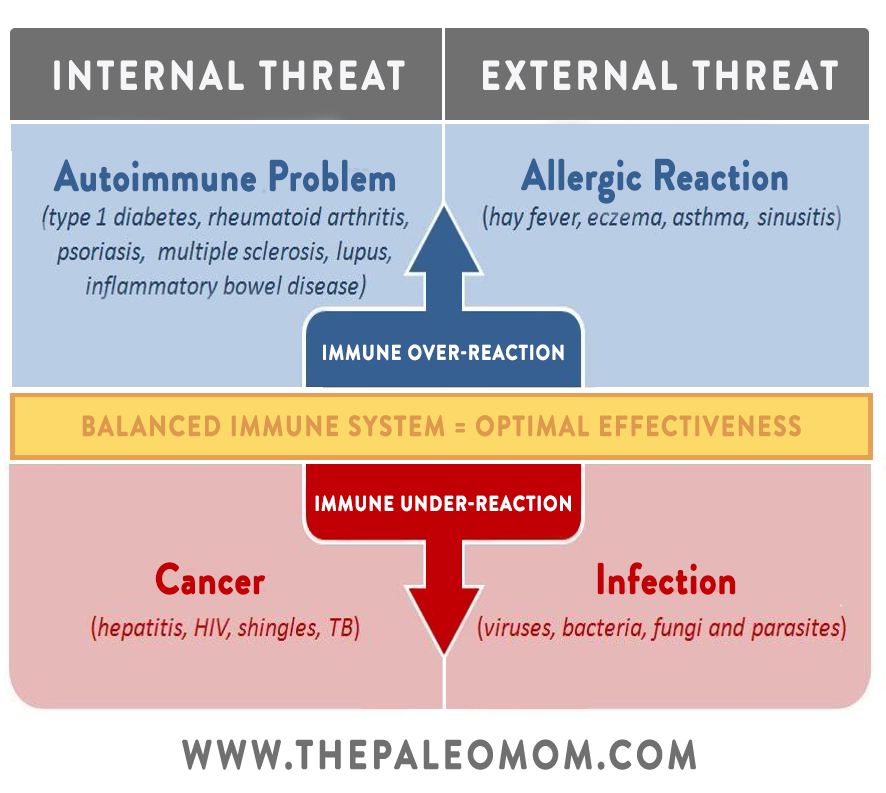
But, sometimes things go wrong! When the delicate balance gets thrown off and we find ourselves in a pattern of Th1 or Th2 dominance, we become more susceptible to specific diseases. In general, Th1 dominance is associated with a variety of autoimmune conditions (including multiple sclerosis, celiac disease, psoriasis, Crohn’s disease, Grave’s disease, Hashimoto’s thyroiditis, type 1 diabetes, rheumatoid arthritis, lichen planus, and psoriasis), while Th2 dominance tends to be associated with allergies, asthma, some systemic autoimmune diseases (like lupus and scleroderma), and cancer. (However, the full story is much more complex than that, and some autoimmune diseases can be either Th1-related or Th2-related depending on other factors. Plus we now know that managing autoimmune disease is far more complex than restoring Th1/Th2 balance, thanks to other T-helper cell subsets like Th9, Th17, and Th22 (to learn more, check out the AIP Lecture Series and The Paleo Approach)).
Now, here’s where the cancer link comes in. Due to its cytokine secretion patterns, the Th1 pathway can both increase autoimmunity and increase the destruction of cancer cells (due to the formation of auto-antibodies that target the body’s own healthy tissue, but also antibodies that target tumor-specific antigens). Th1 cells help activate another type of lymphocyte, killer T cells, that induce tumor cell death. So, people with Th1 dominance (and/or who have a Th1-related autoimmune disease) should technically have some protection against cancer: the body can be hyper-reactive to its own tissues, but it’s also raring to go destroy cells that become cancerous!
Save 70% Off the AIP Lecture Series!
Learn everything you need to know about the Autoimmune Protocol to regain your health!
I am loving this AIP course and all the information I am receiving. The amount of work you have put into this is amazing and greatly, GREATLY, appreciated. Thank you so much. Taking this course gives me the knowledge I need to understand why my body is doing what it is doing and reinforces my determination to continue along this dietary path to heal it. Invaluable!
Carmen Maier

 Conversely, people with Th2 dominance may have some protection against a number of autoimmune conditions (especially the organ-specific autoimmune diseases associated with Th1 dominance), but they may also be at higher risk of cancer. Because Th2 cells can suppress the activity of Th1 cells, people with Th2 dominance have under-active Th1 responses, potentially hindering the body’s ability to destroy cancer cells. Amazingly, tumors themselves can induce a more severe Th1/Th2 imbalance by producing a variety of factors (such as TGF-beta, interleukin-10, prostaglandins, and gangliosides) that create a shift in the Th1/Th2 balance to suppress Th1 responses and support the Th2 pathway. In that way, cancer is able to tweak the host’s immune system to its advantage. (Not surprisingly, clinical studies of cancer patients typically show they have a Th2-dominant profile, and additional research shows that the imbalance tends to increase as the cancer progresses to more advanced stages—reinforcing the idea that the Th1/Th2 imbalance could be an important mechanism for cancer implanting and growing.)
Conversely, people with Th2 dominance may have some protection against a number of autoimmune conditions (especially the organ-specific autoimmune diseases associated with Th1 dominance), but they may also be at higher risk of cancer. Because Th2 cells can suppress the activity of Th1 cells, people with Th2 dominance have under-active Th1 responses, potentially hindering the body’s ability to destroy cancer cells. Amazingly, tumors themselves can induce a more severe Th1/Th2 imbalance by producing a variety of factors (such as TGF-beta, interleukin-10, prostaglandins, and gangliosides) that create a shift in the Th1/Th2 balance to suppress Th1 responses and support the Th2 pathway. In that way, cancer is able to tweak the host’s immune system to its advantage. (Not surprisingly, clinical studies of cancer patients typically show they have a Th2-dominant profile, and additional research shows that the imbalance tends to increase as the cancer progresses to more advanced stages—reinforcing the idea that the Th1/Th2 imbalance could be an important mechanism for cancer implanting and growing.)
In short, autoimmunity and cancer both arise from immune system dysfunction. But, the way that dysfunction occurs is at opposite ends of the spectrum. With autoimmune disease, the body attacks itself when it shouldn’t; with cancer, the body doesn’t attack itself when it should! So, with some notable exceptions (particularly the autoimmune diseases that tend to be associated with Th2 dominance), people who have an autoimmune disease should have some degree of protection against cancer, while people with cancer should be less likely to develop an autoimmune disease. Pretty unfortunate trade-off either way, right?
But wait! There’s just one problem. In real-world human studies, we do see an inverse relationship between some autoimmune diseases and some cancers… but we also see positive relationships showing up fairly frequently (even with the Th1-associated autoimmune conditions that should be cancer-protective). Given the polarity of their immune system dysfunctions, it might seem surprising that these conditions can occur at the same time. Once again, there’s more to the story!
Cancer and Autoimmune Disease Comorbidity: What’s Going On?
In order to understand the paradox of why autoimmunity can decrease cancer risk but also raise the risk of some cancers at the same time, let’s turn the spotlight to everyone’s favorite “I” word… inflammation! Or, more specifically, the ongoing localized inflammation associated with active autoimmune disease.
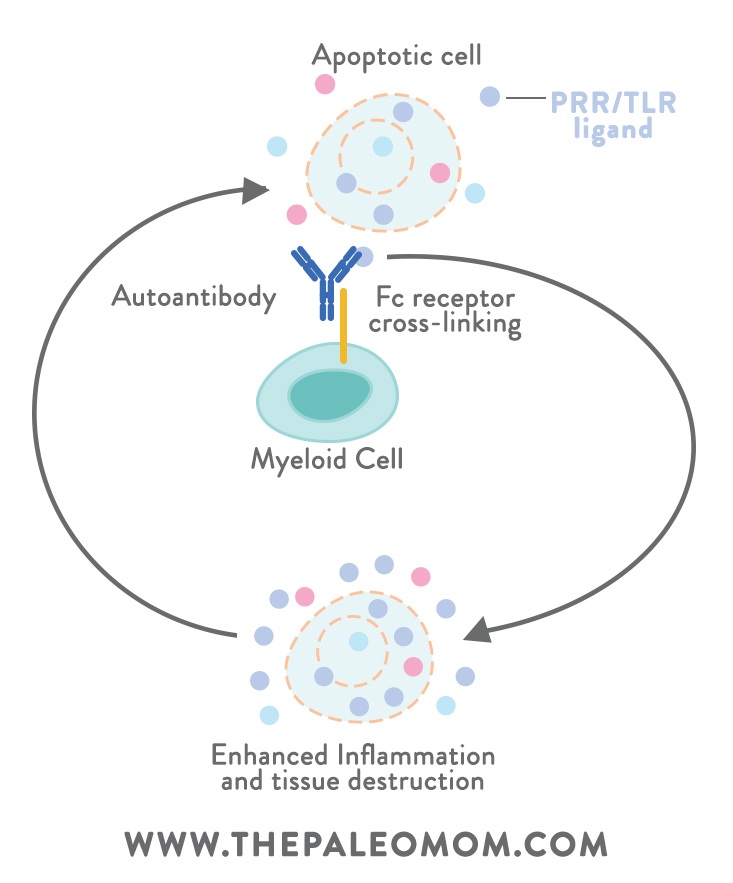
First, let’s back up. Despite getting depicted as a bad guy in most media headlines and health articles, when inflammation is well-regulated, it’s actually beneficial to us: it helps restore tissue injuries, fight infection, and promote healing (if our bodies couldn’t induce inflammation, we’d be toast!). The problem is with chronic inflammation, the kind that doesn’t simply let up after an acute injury is healed. Chronic inflammation is a hallmark of autoimmunity, where it can turn into a vicious self-perpetuating cycle (inflammation from autoimmune reactions causes cell death and the release of endogenous PRR or TLR ligands, which then get recognized by autoantibodies and activate myeloid immune cells, which induce an enhanced inflammatory response, which leads to further tissue destruction and ligand release, which triggers more inflammation to assist with injury repair… on and on it goes!). Unlike immune responses against viruses and other true foreign invaders, the body’s immune response against itself will typically never be complete (since the antigens under attack belong to our own tissue and can’t actually be eliminated!). The result is a sustained immune response and inflammatory damage to tissues.
Here’s why that’s important. When inflammation becomes persistent (as in active autoimmune disease), inflammatory mediators (including free radicals, cytokines, chemokines, and metabolites of arachidonic acid) can act upon local tissues to increase cell proliferation, mutagenesis (genetic changes in a cell), oncogene activation (oncogenes are genes that can transform normal cells into tumor cells), and angiogenesis (the growth of new blood vessels, which can then be used to feed tumors). These changes create the opportunity for rapidly growing and dividing cells to take over nearby tissue. Yep, that’s the perfect setup for cancer! So, we can see how autoimmune disease could theoretically increase cancer risk in the inflammation-affected areas of the body.
In the medical world, there are plenty of examples of inflammation-related cancers, including stomach cancer from H. pylori infection, cervical cancer from papillomavirus infection, and liver cancer from hepatitis B and C infection (in each case, the infection leads to ongoing inflammatory responses that can promote malignancies). Likewise, an autoimmunity-inflammation-cancer connection seems to bear out in the scientific literature as well. Numerous studies show that the cancers associated with autoimmunity often occur in the organ systems or tissues targeted by the autoimmune disease in question. For example, in 2010, a massive study of 4.5 million male veterans in the US (over 96,000 who developed gastrointestinal tract cancers during the course of the follow-up) found that those who had a history of autoimmune disease specifically impacting the GI tract were at much higher risk of cancer in the affected organs (for example, people with a history of ulcerative colitis had a twofold-higher risk of small intestine, colon, and rectal cancers). A literature review spanning a decade’s worth of research found that celiac disease increased the risk of small bowel cancer, colon cancer, colorectal cancer, rectal cancer, anal cancer, liver cancer, and pancreatic cancer. (In addition, celiac disease has been linked with enteropathy-associated T-cell lymphoma that’s localized to the intestine, but the risk goes down with adherence to a gluten-free diet and subsequent mucosal healing!) Studies of Graves disease and Hashimoto’s thyroiditis (both of which affect the thyroid) generally find a significantly higher risk of thyroid cancer in people with these conditions. Other research suggests that multiple sclerosis comes with an overall lower risk of cancer (especially GI tract cancers), but it may increase the risk of central nervous system cancers.
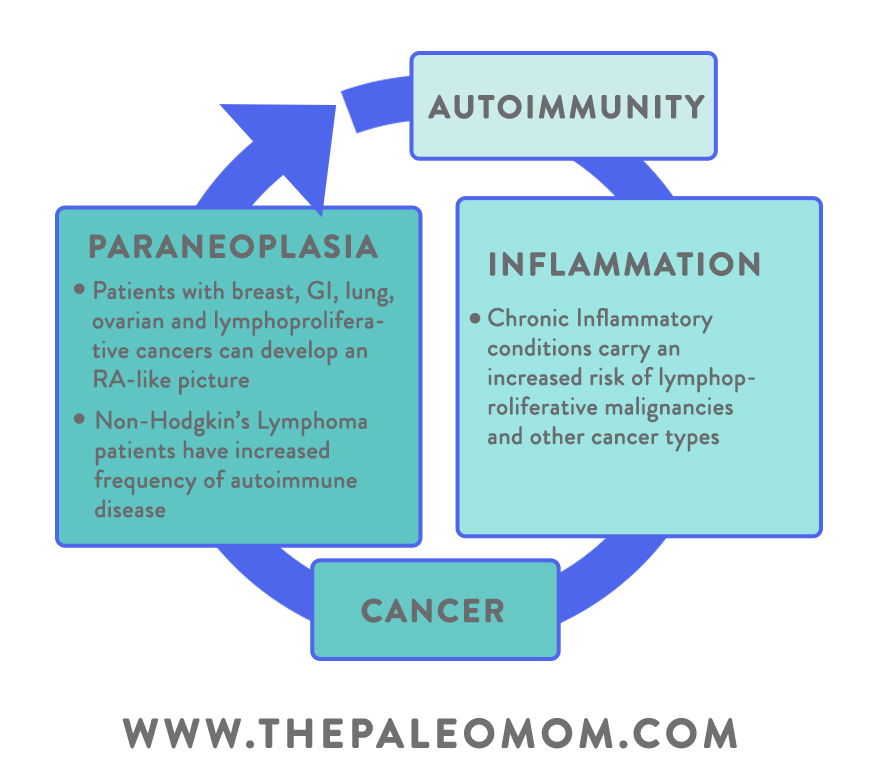
In contrast to organ-specific autoimmune diseases, SARDs (systemic autoimmune rheumatic diseases), which impact multiple organs or tissues at the same time, have their own unique set of cancer patterns. SARDs include rheumatoid arthritis, lupus, Sjögren syndrome, and sclerosis (among others!). One of the unique features of many SARDs is a higher risk of hematological malignancies, AKA cancers that begin in the blood and lymph system. In particular, certain types of lymphoma (including non-Hodgkin lymphoma, or NHL) tend to be more common in people with SARDs, especially when their disease has high cumulative activity levels (for example, one study found that among rheumatoid arthritis patients, those with the highest disease activity had a 60-fold increased risk of lymphoma compared to people with the lowest disease activity).
Although scientists are still exploring the mechanisms behind this link, one theory is that autoimmunity could lead to both the overstimulation and the defective apoptosis (programmed death) of B lymphocytes, allowing them to grow and divide out of control (and potentially becoming B-cell cancers like NHL). It’s also possible that autoimmunity and lymphomas share a common root cause, such as genetic factors and certain viral infections (like Epstein-Barr, which persists in B cells). Despite the exact mechanisms being up for debate, a number of large epidemiological studies show a clear link between the severity of autoimmune activity and subsequent lymphoma risk, suggesting this may play a major role in the cancer’s development.
Sometimes, the complications of autoimmune diseases are associated with cancer as well. For example, interstitial lung disease (or ILD) is a complication of some autoimmune diseases (such as lupus and sclerosis), and is also a risk factor for lung cancer. It involves the infiltration of inflammatory cells into the lungs’ interstitium (the lacy tissue network that supplies support to the lungs’ air sacs). The ongoing inflammation and scarring resulting from ILD could induce DNA damage, contributing to the unexpected connection we see between some autoimmune diseases and lung cancer (in general, the autoimmune conditions that exhibit ILD as a complication are the same ones that are associated with higher lung cancer risk!). Indeed, the scientific literature shows that where ongoing localized inflammation occurs, cancer becomes more likely.
 Just as having an autoimmune disease can raise the risk of certain cancers, having cancer appears to raise the risk of certain autoimmune diseases. In some cases, the connection is extremely logical. For example, vitiligo (a disease that causes the loss of pigment cells on the skin, thought to be autoimmune in origin) is often found in malignant melanoma, and may result from the immune system’s success in targeting cancer cells. Due to similarities between the antigens expressed by melanoma and the antigens expressed by healthy melanocytes (pigment cells), the autoimmunity against melanocytes actually indicates that the body is doing a good job fighting the cancer! (In fact, studies have shown that developing vitiligo during melanoma corresponds with a better prognosis.)
Just as having an autoimmune disease can raise the risk of certain cancers, having cancer appears to raise the risk of certain autoimmune diseases. In some cases, the connection is extremely logical. For example, vitiligo (a disease that causes the loss of pigment cells on the skin, thought to be autoimmune in origin) is often found in malignant melanoma, and may result from the immune system’s success in targeting cancer cells. Due to similarities between the antigens expressed by melanoma and the antigens expressed by healthy melanocytes (pigment cells), the autoimmunity against melanocytes actually indicates that the body is doing a good job fighting the cancer! (In fact, studies have shown that developing vitiligo during melanoma corresponds with a better prognosis.)
In other cases, the way cancer induces autoimmunity is little less obvious. Conditions called paraneoplastic syndromes are known to occur with cancer, and arise due to cross-reactions with tumor antigens. Some tumor antigens resemble proteins in various healthy body tissues (often in areas far removed from where the cancer is occurring!), causing the immune system to lose self-tolerance for those proteins and begin attacking the associated tissue. For example, some breast cancers and ovarian cancers express antigens that mimic neural proteins, leading immune cells to attack the brain’s cerebellar tissue (a condition called paraneoplastic cerebellar degeneration, which can cause loss of motor control). A subset of lupus called subacute cutaneous lupus erythematosus has been found in small-cell lung cancer patients. Additionally, people with malignant tumors or lymphomas can develop a condition resembling rheumatoid arthritis (called carcinomatous polyarthritis), which resolves when the underlying cancer is treated.
In general, it seems relatively more common for autoimmunity to raise the risk of cancer than for cancer itself to raise the risk of autoimmunity, but both scenarios do occur (keeping in mind that “relatively more common” is still generally a small risk, especially if we effectively manage our autoimmune disease). However, another factor influencing the cancer-autoimmunity link has nothing to do with the diseases themselves: it’s the drugs used to treat them!
The Role of Drug Therapy
Because drug therapies for both autoimmune disease and cancer work by manipulating the immune system (again, in very opposite ways due to the polarized etiology behind these conditions), could treating one disease inadvertently raise the risk of another? This question has been asked and studied by a number of researchers over the years! In theory, the answer would seem like a simple “Yes!”, but in reality, it’s much more complex (notice a theme here?!).
Disease Modifying Drugs and Cancer Risk
Let’s start by looking at the drugs used to manage autoimmunity. To counter the inappropriate self-attack seen in autoimmune disease, drug treatments are often designed to calm down inflammation and reduce tissue-damaging immune reactions. Immunosuppressants (like methotrexate), corticosteroids (like prednisone), and “biologics” (such as drugs targeting tumor necrosis factor) are commonly prescribed for this purpose.
The effects of immune-modulating therapies on cancer risk are a mixed bag. In people without autoimmune disease, immunosuppressants are often found to increase cancer risk (for example, when used in conjunction with organ transplants), but existing autoimmunity makes the situation more complicated. On one hand, suppressing autoimmune-related inflammation takes away a major risk factor for cancer development (as we saw earlier, localized inflammation can increase the chances of cancer occurring in that area!). But, suppressing the body’s immune responses also interferes with the ability to detect and destroy malignant cells (which is one of the “perks” of having an over-active immune system!). On a mechanistic level, it’s hard to say which of those effects is more powerful, and whether the net result would encourage cancer growth or protect against it in an autoimmune setting.
Not surprisingly, research gives inconsistent results regarding how immune-modulating drugs impact cancer risk. For example, a number of studies have looked at lymphoma in relation to autoimmune disease to determine whether the drugs used for autoimmune therapy might be encouraging lymphoma development. Among rheumatoid arthritis patients with high disease activity, steroids (whether ingested orally or injected in joints) are associated with lower risk of lymphoma, suggesting that controlling the inflammation is more helpful than allowing immune responses to continue at full-throttle. But, a recent study of biologics (genetically engineered drugs that help block cytokines) in people with early rheumatoid arthritis found that drug use didn’t affect the risk of developing lymphomas (though it did decrease the risk of other cancers!). And, certain chemotherapeutic disease-modifying drugs, particularly cyclophosphamide, appear to significantly increase the risk of lymphoma (along with leukemia and bladder cancers) in both rheumatoid arthritis and lupus. (In contrast to the protective effect of steroids in rheumatoid arthritis, high cumulative steroid exposure in lupus raises lymphoma risk.) Confusing, right?
In addition, certain autoimmune treatments may specifically raise the risk of skin cancer. For example, some research has found an increase in non-melanoma skin cancer when thiopurine is used to treat IBD, when methotrexate or anti-TNF therapies are used to treat rheumatoid arthritis, and when PUVA, cyclosporine, and anti-TNF therapies are used to treat psoriasis. Meanwhile, in multiple sclerosis, immunosuppressant therapy appears to have a direct, strong, risk-raising effect for cancer in general (related more to the duration and cumulative dose of the immunosuppressant than the specific type of immunosuppressant used). Yep, the research is all over the map!
To complicate things further, it can be hard to tease apart the pro-cancer effects of drugs from the pro-cancer effects of the autoimmune diseases themselves. Typically, immunosuppressant usage goes up when disease activity is higher, so when an increased risk pops up between drug exposure and cancer in studies, we can’t always tell whether it’s due to the treatment or due to the severity of the disease.
Lastly, for many drug and autoimmune disease combinations, we don’t yet have sufficient long-term evidence to draw conclusions about cancer risk (especially since that risk can vary between genders, age groups, disease status during drug exposure, and a huge list of other variables that require large study populations to assess). Combined with the mixed findings from existing studies, it’s impossible to make any blanket statements about the effect of autoimmune drug therapies on subsequent cancer risk. The best we can do is evaluate each situation on a case-by-case basis to determine whether medication, and what kind, will do more benefit than harm.
Cancer Treatments and Autoimmune Disease
On the flip side, certain cancer therapies have been associated with the development of autoimmunity. In breast cancer, the use of aromatase inhibitors (such as letrozole) can induce a form of lupus called subacute cutaneous lupus erythematosus, which causes non-scarring plaques on sunlight-exposed parts of the skin. Due to their estrogen-lowering effect, aromatase inhibitors ramp up the function of neutrophils (which are inhibited by estrogen), allowing them to adhere to the blood vessel endothelium and provoke an autoimmune inflammatory reaction (vasculitis). Similar autoimmune reactions have happened after using tamoxifen or receiving docetaxel chemotherapy for breast cancer.
Newly approved cancer drugs called immune checkpoint inhibitors (ICIs) are also being studied for their potential to induce autoimmunity. These drugs work by non-specifically activating T cells, which can help the body fight malignancy but also raises the risk of antibody formation against healthy tissue. Among cancer patients with an existing autoimmune disease, ICIs have been shown to both induce flare-ups and create new immune-related rheumatic events (but, their newness to the market means there isn’t a whole lot of research yet on their autoimmune effects!).
Recently, a study of over 20,000 childhood cancer survivors found they had a significantly increased risk of developing an autoimmune disease later in life (including haemolytic anemia, Sjögren syndrome, chronic rheumatic heart disease, and Hashimoto’s thyroiditis). Although some of that risk could be due to long-lasting immune changes from the cancer itself, the researchers noted that chemotherapy can cause persistent immune abnormalities that lead to autoantibody formation, and that other forms of cancer treatment like radiotherapy might also play a role in autoimmunity (though much more research is needed to understand how it works!).
Reducing Cancer Risk for Autoimmune Disease Sufferers
I know, I know: those of us with autoimmune diseases already have enough to worry about… do we really need to add cancer to that list?! Ultimately, it’s in our own best interest to be aware of how our existing health conditions (and when applicable, the medications we take for them) could be making us more susceptible to other diseases, so that we can minimize our controllable risk factors and detect any new conditions early on. But, it’s also important to remember that “increased risk” isn’t the same as saying we’ll definitely get cancer! It just means we should be on the alert for new symptoms, stick with health-promoting diet and lifestyle behaviors, and get cancer screenings and lab work by medical professionals when necessary. Needless worry doesn’t help anybody, but vigilance can save lives!
One of the problems that autoimmune patients face is the fact that symptoms of cancer can be similar to the symptoms of autoimmune diseases, leading cancer to initially go unnoticed. A study in Sweden found that, while MS patients were less likely to develop cancer than the general population, the ones who did develop cancer tended to have larger tumor sizes at the time of diagnosis. This could be because fatigue and other characteristics of MS resemble the symptoms of cancer, so when they occur, MS patients are less likely to suspect something else is wrong and get checked out at the doctor. Moral of the story, if we have an active autoimmune disease, we should avoid dismissing any new or worsening symptoms as part of our condition and instead rule out other possibilities. Again, better safe than sorry!
Likewise, many studies have linked cancer risk specifically to higher autoimmune disease activity… which means it’s really important to do everything in our power to manage our condition and reduce flare-ups! That means following the Autoimmune Protocol, identifying our personal diet and lifestyle triggers, getting plenty of sleep, reducing stress, maintaining a healthy level of physical activity (neither too much nor too little), and taking time to rest and recuperate if we’ve been pushing ourselves too hard. For people with celiac disease, strict adherence to a gluten-free diet can almost completely reverse the excess cancer risk (just something to remember if you find yourself thinking “this one bite of cookie won’t hurt!”). To learn everything you need to know about regulating immune function for those with autoimmune disease using diet and lifestyle, check out the AIP Lecture Series.
When it comes to drug therapy, the science is much more of a gray zone. Our best approach here is to research the cancer risks associated with any medications we’re taking or are considering taking, consult a medical professional, and evaluate the pros and cons. For some autoimmune diseases, like rheumatoid arthritis, keeping disease activity low seems to outweigh the risks of immune-suppressing drug side effects—but again, every situation is different!
Once again, the relationship between autoimmunity and cancer is complex and still not fully understood. While scientists continue to explore the mechanisms between these diseases (which, in turn, will give us more information on how to reduce our risk!), staying vigilant about symptoms and managing our conditions through proper diet and lifestyle habits is our best bet!
Citations
Albert ML & Darnell RB. “Paraneoplastic neurological degenerations: keys to tumour immunity.” Nat Rev Cancer. 2004 Jan;4(1):36-44.
Archontogeorgis K, et al. “Lung cancer and interstitial lung diseases: a systematic review.” Pulm Med. 2012;2012:315918. doi: 10.1155/2012/315918. Epub 2012 Jul 29.
Baecklund E, et al. “Lymphoma development in patients with autoimmune and inflammatory disorders–what are the driving forces?” Semin Cancer Biol. 2014 Feb;24:61-70. doi: 10.1016/j.semcancer.2013.12.001. Epub 2013 Dec 10.
Berger A. “Th1 and Th2 responses: what are they?” BMJ. 2000 Aug 12; 321(7258): 424.
Bernatsky S, et al. “Lymphoma risk in systemic lupus: effects of disease activity versus treatment.” Ann Rheum Dis. 2014 Jan;73(1):138-42. doi: 10.1136/annrheumdis-2012-202099. Epub 2013 Jan 8.
Bernatsky S, et al. “Non-Hodgkin’s lymphoma–meta-analyses of the effects of corticosteroids and non-steroidal anti-inflammatoriesß.” Rheumatology (Oxford). 2007 Apr;46(4):690-4. Epub 2006 Dec 5.
Berstein CN, et al. “Cancer risk in patients with inflammatory bowel disease: a population-based study.” Cancer. 2001 Feb 15;91(4):854-62.
Beyaert R, et al. “Cancer risk in immune-mediated inflammatory diseases (IMID).” Mol Cancer. 2013 Aug 29;12(1):98. doi: 10.1186/1476-4598-12-98.
Byrne KT, et al. “Autoimmune melanocyte destruction is required for robust CD8+ memory T cell responses to mouse melanoma.” J Clin Invest. 2011 May;121(5):1797-809. doi: 10.1172/JCI44849. Epub 2011 Apr 11.
“Cancer Facts & Figures 2017.” American Cancer Society. https://www.cancer.org/content/dam/cancer-org/research/cancer-facts-and-statistics/annual-cancer-facts-and-figures/2017/cancer-facts-and-figures-2017.pdf
Cappelli LC, et al. “Cancer immunotherapy-induced rheumatic diseases emerge as new clinical entities.” RMD Open. 2016 Sep 28;2(2):e000321. eCollection 2016.
Caspi R. “Immunotherapy of autoimmunity and cancer: the penalty for success.” Nat Rev Immunol. 2008 Dec; 8(12): 970–976.
Cho SK, et al. “The risk of malignancy and its incidence in early rheumatoid arthritis patients treated with biologic DMARDs.” Arthritis Res Ther. 2017 Dec 15;19(1):277. doi: 10.1186/s13075-017-1482-y.
Deivendran S, et al. “The role of inflammation in cervical cancer.” Adv Exp Med Biol. 2014;816:377-99. doi: 10.1007/978-3-0348-0837-8_15.
Dias C & Isenberg DA. “Susceptibility of patients with rheumatic diseases to B-cell non-Hodgkin lymphoma.” Nat Rev Rheumatol. 2011 Jun;7(6):360-8. doi: 10.1038/nrrheum.2011.62.
Evans KG & Heymann WR. “Paraneoplastic subacute cutaneous lupus erythematosus: an underrecognized entity.” Cutis. 2013 Jan;91(1):25-9.
Fallah M, et al. “Autoimmune diseases associated with non-Hodgkin lymphoma: a nationwide cohort study.” Ann Oncol. 2014 Oct;25(10):2025-30. doi: 10.1093/annonc/mdu365. Epub 2014 Jul 31.
Franks AL & Slansky JE. “Multiple Associations Between a Broad Spectrum of Autoimmune Diseases, Chronic Inflammatory Diseases and Cancer.” Anticancer Res. 2012 Apr;32(4):1119-1136.
Freeman HJ. “Colorectal cancer risk in Crohn’s disease.” World J Gastroenterol. 2008 Mar 28;14(12):1810-1811.
Fumal I, et al. “Subacute cutaneous lupus erythematosus associated with tamoxifen therapy: two cases.” Dermatology. 2005;210(3):251-2.
Gharia B, et al. “Letrozole-induced hepatitis with autoimmune features: a rare adverse drug reaction with review of the relevant literature.” Oxf Med Case Reports. 2017 Nov 13;2017(11):omx074. doi: 10.1093/omcr/omx074. eCollection 2017 Nov.
Giat E, et al. “Cancer and autoimmune diseases.” Autoimmun Rev. 2017 Oct;16(10):1049-1057. doi: 10.1016/j.autrev.2017.07.022. Epub 2017 Aug 1.
Holmqvist AS, et al. “Autoimmune diseases in Adult Life after Childhood Cancer in Scandinavia (ALiCCS).” Ann Rheum Dis. 2016 Sep;75(9):1622-9. doi: 10.1136/annrheumdis-2015-207659. Epub 2015 Nov 10.
Islam MS, et al. “A case of anastrazole-related drug-induced autoimmune hepatitis.” Clin J Gastroenterol. 2014 Oct;7(5):414-7. doi: 10.1007/s12328-014-0512-4. Epub 2014 Jul 22.
Kopylov U, et al. “Risk of Lymphoma, Colorectal and Skin Cancer in Patients with IBD Treated with Immunomodulators and Biologics: A Quebec Claims Database Study.” Inflamm Bowel Dis. 2015 Aug;21(8):1847-53. doi: 10.1097/MIB.0000000000000457.
Landgren AM, et al. “Autoimmune disease and subsequent risk of developing alimentary tract cancers among 4.5 million US male veterans.” Cancer. 2011 Mar 15;117(6):1163-71. doi: 10.1002/cncr.25524. Epub 2010 Nov 2.
Nielsen NM, et al. “Cancer risk among patients with multiple sclerosis: a population-based register study.” Int J Cancer. 2006 Feb 15;118(4):979-84.
Okada F. “Inflammation-related carcinogenesis: current findings in epidemiological trends, causes and mechanisms.” Yonago Acta Med. 2014 Jun;57(2):65-72. Epub 2014 Jul 30.
Radis CD, et al. “Effects of cyclophosphamide on the development of malignancy and on long-term survival of patients with rheumatoid arthritis. A 20-year followup study.” Arthritis Rheum. 1995 Aug;38(8):1120-7.
Ragonese P, et al. “Association between multiple sclerosis, cancer risk, and immunosuppressant treatment: a cohort study.” BMC Neurol. 2017 Aug 8;17(1):155. doi: 10.1186/s12883-017-0932-0.
Romagnani S. “T-cell subsets (Th1 versus Th2).” Ann Allergy Asthma Immunol. 2000 Jul;85(1):9-18; quiz 18, 21.
Shurin MR, et al. “Th1/Th2 balance in cancer, transplantation and pregnancy.” Springer Semin Immunopathol. 1999;21(3):339-59.
Smedby KE, et al. “Autoimmune and inflammatory disorders and risk of malignant lymphomas–an update.” J Intern Med. 2008 Dec;264(6):514-27. doi: 10.1111/j.1365-2796.2008.02029.x.
Suurmond J & Diamond B. “Autoantibodies in systemic autoimmune diseases: specificity and pathogenicity.” J Clin Invest. 2015 Jun;125(6):2194-202. doi: 10.1172/JCI78084. Epub 2015 May 4.
Świerkot J, et al. “[Tumors in rheumatic diseases].” Postepy Hig Med Dosw (Online). 2013 Dec 11;67:1254-60.
Turesson C & Matteson EL. “Malignancy as a comorbidity in rheumatic diseases.” Rheumatology (Oxford). 2013 Jan;52(1):5-14. doi: 10.1093/rheumatology/kes189. Epub 2012 Jul 23.
Wilton KM & Matteson EL. “Malignancy Incidence, Management, and Prevention in Patients with Rheumatoid Arthritis.” Rheumatol Ther. 2017 Dec;4(2):333-347. doi: 10.1007/s40744-017-0064-4. Epub 2017 May 15.
Wong NY, et al. “Drug-induced subacute cutaneous lupus erythematosus associated with docetaxel chemotherapy: a case report.” BMC Res Notes. 2014 Nov 5;7:785. doi: 10.1186/1756-0500-7-785.
Wu Y & Hou Q. “Systemic lupus erythematous increased lung cancer risk: Evidence from a meta-analysis.” J Cancer Res Ther. 2016 Apr-Jun;12(2):721-4. doi: 10.4103/0973-1482.172115.
Yu KH, et al. “Cancer Risk in Patients With Inflammatory Systemic Autoimmune Rheumatic Diseases: A Nationwide Population-Based Dynamic Cohort Study in Taiwan.” Medicine (Baltimore). 2016 May;95(18):e3540. doi: 10.1097/MD.0000000000003540.
Zarkavelis G, et al. “Aromatase inhibitors induced autoimmune disorders in patients with breast cancer: A review.” J Adv Res. 2016 Sep;7(5):719-726. doi: 10.1016/j.jare.2016.04.001. Epub 2016 Apr 23.
Zintzaras E, et al. “The risk of lymphoma development in autoimmune diseases: a meta-analysis.” Arch Intern Med. 2005 Nov 14;165(20):2337-44.
Zupancic M, et al. “Migratory polyarthritis as a paraneoplastic syndrome.” J Gen Intern Med. 2008 Dec;23(12):2136-9. doi: 10.1007/s11606-008-0794-7. Epub 2008 Sep 23.