Glutamine is the most abundant amino acid in our body, and it also plays a major role in the health and function of the gut. In fact, ample research shows that glutamine influences our gut health in two important ways: one, through its effects on the gut barrier, and secondly, through its effects on our gut microbiota! Let’s take a look at how this awesome amino acid works its magic through these two avenues.
Table of Contents[Hide][Show]
Glutamine and the Gut Barrier
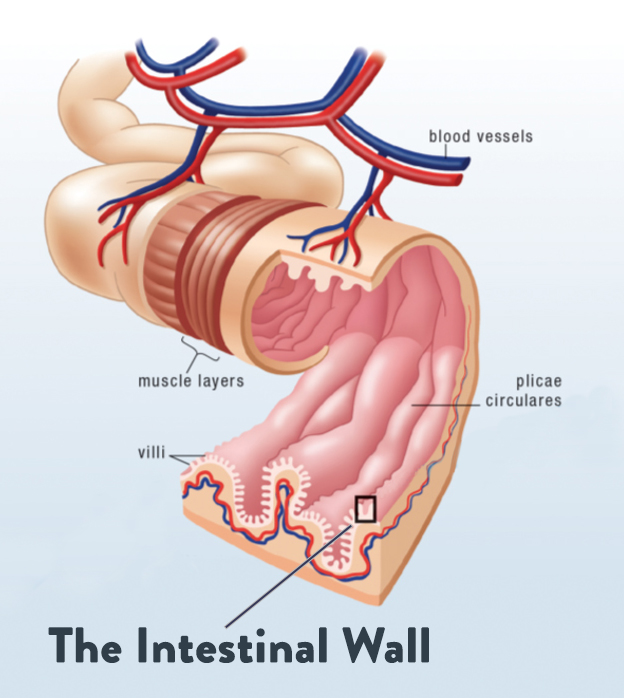 The gut barrier consists of a single layer of firmly connected cells lining the intestine, serving as a barrier between the external world (our digestive tract, which is technically a tube that runs “outside” our body!) and our internal environment (the inside of our body). When this barrier is functioning properly, it prevents foreign antigens, pathogens, and bacterial toxins from entering our system, while also selectively allowing essential nutrients and water to filter through. When this barrier isn’t functioning properly (as is the case with leaky gut), protein fragments, endotoxin, waste, pathogens, and other harmful substances can leave the gut and enter circulation. In that situation, immune cells in the gut launch an attack on the foreign invaders, leading to a cascade of immune reactions and ongoing inflammation. In short, gut barrier function is essential for keeping us healthy, and when it’s in a compromised state, so are we! (For more on the gut barrier and how leaky gut develops, check out “What Is A Leaky Gut?”, as well as my Gut Health Fundamentals online course.)
The gut barrier consists of a single layer of firmly connected cells lining the intestine, serving as a barrier between the external world (our digestive tract, which is technically a tube that runs “outside” our body!) and our internal environment (the inside of our body). When this barrier is functioning properly, it prevents foreign antigens, pathogens, and bacterial toxins from entering our system, while also selectively allowing essential nutrients and water to filter through. When this barrier isn’t functioning properly (as is the case with leaky gut), protein fragments, endotoxin, waste, pathogens, and other harmful substances can leave the gut and enter circulation. In that situation, immune cells in the gut launch an attack on the foreign invaders, leading to a cascade of immune reactions and ongoing inflammation. In short, gut barrier function is essential for keeping us healthy, and when it’s in a compromised state, so are we! (For more on the gut barrier and how leaky gut develops, check out “What Is A Leaky Gut?”, as well as my Gut Health Fundamentals online course.)
So, where does glutamine come into play?
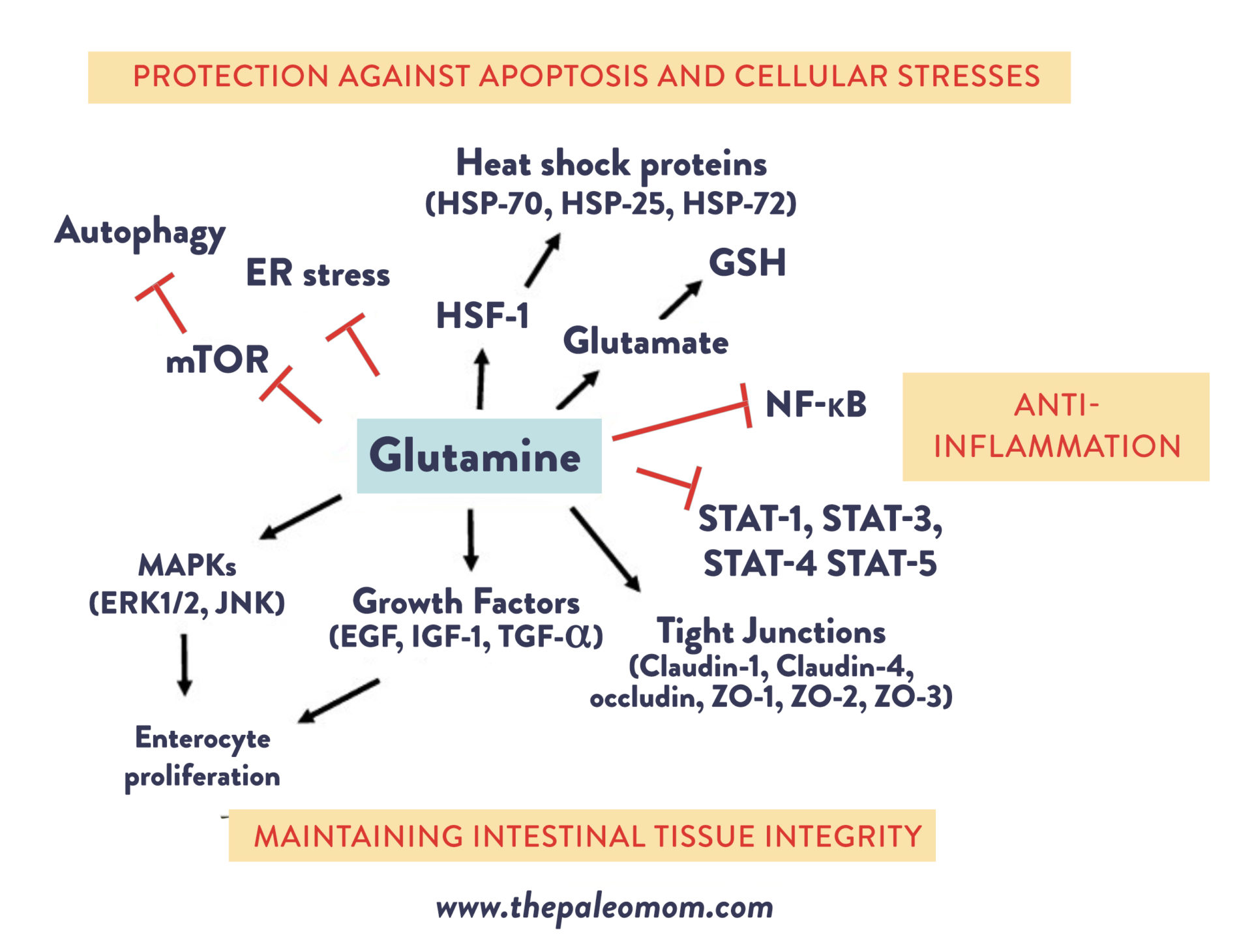
For one, glutamine is the preferred fuel source for cells lining the gut. In fact, the intestinal mucosa uses about 30% of the body’s total glutamine, demanding more than 15 grams of it per day! Our intestine actually competes with other tissues in our body for use of glutamine from our food and from our body’s amino acid pool! Glutamine is needed in order for those intestinal cells to grow and differentiate (mature), and does so by activating mitogen-activated protein kinases (MAPKs; which orchestrate cell proliferation and differentiation), and by augmenting the effects of growth factors (like epidermal growth factor, transforming growth factor-α, and insulin-like growth factor-I). In vitro studies have shown that, without enough glutamine, two types of epithelial cells (caco-2, a line of human epithelial colon cancer cells that is widely used to study tight junctions and which I used to use for cell culture studies way back in my cell biology research days, and IEC-6) exhibit slowed growth. Likewise, glutamine supplementation has been shown to decrease mucosal atrophy and reduce oxidant injury in models of ulcerative colitis, due to its role in supporting intestinal epithelial cell growth!
 Secondly, glutamine modulates the expression of tight junction proteins, which tightly join together epithelial cells to create the gut barrier. Experiments have shown that glutamine deprivation reduces the expression of several different tight junction proteins (including claudin-1, ZO-1, and occludin, all of which I used to study, so just writing this is bringing back memories of hours and hours in the confocal microscopy suite), significantly increases epithelial cell permeability (AKA leaky gut), and results in villous atrophy (when the intestinal villi erode away, like what happens in celiac disease). And, adding glutamine to glutamine-deprived cells has been shown to improve the barrier function, increase the expression of ZO-1 and occludin proteins (indicating the formation of tighter tight junctions), and prevent chemically induced disruptions of the tight junctions and their permeability. Research using caco-2 cells has shown that glutamine deprivation leads to greater bacterial translocation.
Secondly, glutamine modulates the expression of tight junction proteins, which tightly join together epithelial cells to create the gut barrier. Experiments have shown that glutamine deprivation reduces the expression of several different tight junction proteins (including claudin-1, ZO-1, and occludin, all of which I used to study, so just writing this is bringing back memories of hours and hours in the confocal microscopy suite), significantly increases epithelial cell permeability (AKA leaky gut), and results in villous atrophy (when the intestinal villi erode away, like what happens in celiac disease). And, adding glutamine to glutamine-deprived cells has been shown to improve the barrier function, increase the expression of ZO-1 and occludin proteins (indicating the formation of tighter tight junctions), and prevent chemically induced disruptions of the tight junctions and their permeability. Research using caco-2 cells has shown that glutamine deprivation leads to greater bacterial translocation.
Although the mechanisms aren’t completely understood yet, glutamine appears to influence signaling pathways related to different tight junction components, including the regulation of phosphorylation states of tight junction proteins via the phosphatidylinositol 3-kinase (PI3K)/Akt pathway. On top of that, glutamine may help maintain the balance between proliferation (reproduction) and apoptosis (programmed death) of the epithelial cells lining the intestine. These cells have a rapid turnover rate of every four to five days, and if an imbalance occurs between cellular reproduction versus cellular death, a net loss of epithelial cells can result (which we see in conditions like ulcerative colitis, celiac disease, and intestinal bacterial infection!). A variety of cellular stressors like nutrient deprivation, lack of growth factor, endotoxemia, or gut-harming agents that make their way into our bodies can lead to increased apoptosis. But, glutamine may play a key role in preventing this! Studies have shown that glutamine supplementation suppresses the chemically-induced apoptosis of intestinal epithelial cells, and when there’s a shortage of available glutamine, apoptosis of intestinal cells increases.
Glutamine’s anti-apoptotic properties work through a variety of mechanisms. One is through its ability to modulate cellular stress responses, including autophagy (a catabolic process that occurs during metabolic stress conditions, often referred to as a “Spring cleaning” of cellular organelles) and endoplasmic reticulum stress (which has been shown to trigger sustained apoptosis). Another is via glutamine’s role as a precursor for the antioxidant tripeptide glutathione (GSH), which is key for maintaining normal cellular redox status. When glutathione is depleted, excessive oxidative stress can induce apoptosis! Additionally, glutamine can enhance the expression of heat shock proteins, which appear to modulate programmed cell death by helping cells adapt to stressful conditions. In animal experiments, administering glutamine to rats with sepsis was able to significantly increase the expression of two heat shock proteins (HSP-70, which is my personal favorite heat shock protein because I studied it during my PhD research, and HSP-25), while glutamine deprivation reduced the expression of heat shock protein genes and led to increased cell death. And lastly, glutamine regulates the activation of caspases, a family of protease enzymes that act in concert in a cascade to trigger and execute apoptosis. Studies have shown that glutamine-deprived cells demonstrate higher caspase-3 activity, leading to greater levels of cell death. In animal models and human colon cancer cells, administering glutamine has been shown to reduce caspase activity and protect against apoptosis.
So, in a nutshell, glutamine is a huge boon for our gut barrier due to the many ways it keeps our epithelial cells healthy and proliferating!
But, it doesn’t end there! Glutamine also plays a role in intestinal immunity. In particular, glutamine enhances the secretion of immunoglobulin A+ (IgA+) and intestinal secretory immunoglobulin A (SIgA), which helps defend the intestinal mucosal surface against pathogens and enteric toxins. Glutamine is also needed for the growth of immune cells, and not surprisingly, glutamine insufficiency has been shown to increase susceptibility to infection.
Glutamine and the Gut Microbiota
Along with benefiting our human cells, glutamine can influence the composition of the gut microbiota—the massive community of microbes inhabiting our gut, and which plays a major role in human health. (Be sure to check out What is the Gut Microbiome? And Why Should We Care About It?)
Protein is essential for the growth and survival of the bacteria in our digestive tracks. Amino acids are utilized for the synthesis of bacterial cell components or catabolized (broken apart) through different pathways. Certain amino acids have been identified as likely essential for optimal growth of gut bacteria, including arginine, aspartate, asparagine, glutamate, glutamine (yay!), glycine, lycine, serine, threonine, and the branched-chain amino acids (leucine, isoleucine, and valine). Interestingly, our gut bacteria seem to have a preference for glutamine (and arginine, which I’ll talk about in a future article), which is why it can benefit gut microbial composition.
In a trial of overweight and obese adults, consuming 30 grams of supplemental glutamine each day for two weeks resulted in a beneficial reduction in the Firmicutes to Bacteroidetes ratio (from 0.85 to 0.57), lower levels of Actinobacteria, a change associated with leanness and weight loss. The bacteria genera Dialister, Dorea, Pseudobutyrivibrio, and Veillonella (all members of Firmicutes) also significantly decreased.
One investigation looked at the gut microbiota and a variety of metabolites (including fatty acids, lipids, glucose, and amino acids) in 531 Finnish men. The researchers found that glutamine levels were positively associated with microbial richness, as well as with higher levels of Clostridales.
Animal studies have also shed light on the effects of glutamine on the gut microbiota. In one experiment with mice, supplementing with 1% L-glutamine for 14 days resulted in a beneficial decrease in the Firmicutes to Bacteroidetes ratio. In a rat model of colon cancer, glutamine treatment protected against a chemotherapy-induced decline of Clostridium cluster XI and Enterobacteriaceae. Constipated animals supplemented with glutamine saw an increase in beneficial members of Bacteroidetes and Actinobacteria, which played a role in the amelioration of constipation (more specifically, glutamine appears to reduce constipation by affecting nitrogen balance and protein synthesis in the small intestinal microbiota). A study using pregnant constipated pigs found that supplementing the diet with 10 grams of glutamine was enough to reduce their constipation and caused an increase in friendly bacteria, while decreasing levels of the potentially harmful bacteria Oscillospira and Treponema.
Fascinatingly, glutamine also seems to influence how our intestinal bacteria metabolize other amino acids. In one experiment, the addition of glutamine to mixed bacterial cultures as well as individual bacteria strains (including species from Streptococcus, Escherichia coli, and Klebsiella) reduced the bacterial usage of asparagine, lysine, leucine, valine, ornithine and serine, with higher concentrations of glutamine causing greater decreases in the utilization of these amino acids, called an amino acid sparing effect. This is important, because the metabolism of amino acids by our gut microbes affects how much of them are available for the rest of our body!
How to Get the Benefits of Glutamine
 So, how can we reap the gut-health rewards of glutamine? Although this amino acid is only considered conditionally essential (meaning that under normal circumstances, we can produce enough of it internally), certain situations can increase our demand beyond what our bodies can supply us with. This includes times of stress, critical illness, infection, wound healing, trauma, severe burns, gastrointestinal disorders, and intense exercise (in fact, reduced glutamine concentrations may play a role in the transient immunosuppression we see following strenuous exercise!). In other words, focusing on getting plenty of glutamine isn’t a bad idea!
So, how can we reap the gut-health rewards of glutamine? Although this amino acid is only considered conditionally essential (meaning that under normal circumstances, we can produce enough of it internally), certain situations can increase our demand beyond what our bodies can supply us with. This includes times of stress, critical illness, infection, wound healing, trauma, severe burns, gastrointestinal disorders, and intense exercise (in fact, reduced glutamine concentrations may play a role in the transient immunosuppression we see following strenuous exercise!). In other words, focusing on getting plenty of glutamine isn’t a bad idea!
In our diet, the best sources of glutamine include broth, seafood (especially crustaceans like shrimp, crab, and lobster, but also saltwater fish), organ meats, poultry, pork, red meat, and protein-rich dairy. Certain plant foods like cabbage, asparagus, and broccoli are also rich in glutamine! In general, aiming for a protein intake of 100 to 150 grams per day, including some glutamine-rich foods, can help ensure we get enough of this amazing amino acid to experience its benefits. (See also Why Broth is Awesome, Broth: Hidden Dangers in a Healing Food?, Bone Broth Risks: Skim the Fat!, Why Fish is Great for the Gut Microbiome, Oysters, Clams, and Mussels, Oh My! Nutrition Powerhouses or Toxic Danger?, The Mercury Content of Seafood: Should we worry?, Should We Be Worried About Radiation from Fukushima?, Grass-Fed Beef: A Superfood worth the Premium Price, Why Everyone Should Be Eating Organ Meat).
In some cases, glutamine supplementation can bring added benefits, such as gastrointestinal disorders, gut dysbiosis, and “leaky gut”, whether primary or secondary to something like autoimmune disease. Most successful trials have used supplementation at levels of 20 – 30 grams of glutamine per day. Because amino acids compete with other high-affinity amino acids for binding with their transporters within the intestine, it’s important to take glutamine on an empty stomach (an hour after food, or 30 minutes before food) so that our bodies can fully utilize it!
Citations
Achamrah N, et al. “Glutamine and the regulation of intestinal permeability: from bench to bedside.” Curr Opin Clin Nutr Metab Care. 2017 Jan;20(1):86-91.
Dai ZL, et al. “L-Glutamine regulates amino acid utilization by intestinal bacteria.” Amino Acids. 2013 Sep;45(3):501-12. doi: 10.1007/s00726-012-1264-4. Epub 2012 Mar 24.
de Souza AZ, et al. “Oral supplementation with L-glutamine alters gut microbiota of obese and overweight adults: A pilot study.” Nutrition. 2015 Jun;31(6):884-9. doi: 10.1016/j.nut.2015.01.004. Epub 2015 Jan 29.
Gleeson M, et al. “Dosing and efficacy of glutamine supplementation in human exercise and sport training.” J Nutr. 2008 Oct;138(10):2045S-2049S.
Kim MH & Kim H. “The Roles of Glutamine in the Intestine and Its Implication in Intestinal Diseases.” Int J Mol Sci. 2017 May 12;18(5). pii: E1051. doi: 10.3390/ijms18051051.
Lacey JM & Wilmore DW. “Is glutamine a conditionally essential amino acid?” Nutr Rev. 1990 Aug;48(8):297-309.
Li N, et al. “Glutamine regulates Caco-2 cell tight junction proteins.” Am J Physiol Gastrointest Liver Physiol. 2004 Sep;287(3):G726-33. Epub 2004 May 6.
Morris CR, et al. “Acquired Amino Acid Deficiencies: A Focus on Arginine and Glutamine.” Nutr Clin Pract. 2017 Apr;32(1_suppl):30S-47S. doi: 10.1177/0884533617691250. Epub 2017 Feb 1.
Org E, et al. “Relationships between gut microbiota, plasma metabolites, and metabolic syndrome traits in the METSIM cohort.” Genome Biol. 2017 Apr 13;18(1):70. doi: 10.1186/s13059-017-1194-2.
Perna S, et al. “The Role of Glutamine in the Complex Interaction between Gut Microbiota and Health: A Narrative Review.” Int J Mol Sci. 2019 Oct; 20(20): 5232.
Rao R & Samak G. “Role of Glutamine in Protection of Intestinal Epithelial Tight Junctions
Ren W, et al. “Dietary L-glutamine supplementation modulates microbial community and activates innate immunity in the mouse intestine.” Amino Acids. 2014 Oct;46(10):2403-13. doi: 10.1007/s00726-014-1793-0. Epub 2014 Jul 15.
Rao R & Samak G. “Role of Glutamine in Protection of Intestinal Epithelial Tight Junctions.” J Epithel Biol Pharmacol. 2012 Jan;5(Suppl 1-M7):47-54.
Rhode T, et al. “Glutamine, exercise, and the immune system–is there a link?” Exerc Immunol Rev. 1998;4:49-63.
Wang B, et al. “Glutamine and intestinal barrier function.” Amino Acids. 2015 Oct;47(10):2143-54. doi: 10.1007/s00726-014-1773-4. Epub 2014 Jun 26.
Zhang Y, et al. “L-Glutamine Supplementation Alleviates Constipation during Late Gestation of Mini Sows by Modifying the Microbiota Composition in Feces.” Biomed Res Int. 2017;2017:4862861. doi: 10.1155/2017/4862861. Epub 2017 Mar 12.












