The gut is a biological niche, home to a diverse array of microbes that influence nearly all aspects of human biology through their interactions with our bodies.
 The term gut microbiota refers to the massive collection of microorganisms that inhabit our gastrointestinal tract. And “massive” is far from hyperbole: an estimated 30-100 trillion bacteria (along with fungi, viruses, and archaea) comprise the microbiota, collectively weighing around 4.5 pounds and containing over 150 times more genes than our own human genome! These microbes include a mixture of commensal (neutrally existing), symbiotic (mutually beneficial), and pathogenic (harmful to us) organisms, and can consist of any of 35,000 species known to inhabit the human gut. Every person’s gut contains approximately 400 to 1,500 different species of the possible 35,000 different microorganisms that are well adapted to survive in the gastrointestinal tract, although about 99 percent of those microorganisms come from thirty to forty species of bacteria. Our guts are inhabited by other microorganisms besides bacteria, including archaea (similar to bacteria), viruses, and single-cell eukaryotes (like yeast).
The term gut microbiota refers to the massive collection of microorganisms that inhabit our gastrointestinal tract. And “massive” is far from hyperbole: an estimated 30-100 trillion bacteria (along with fungi, viruses, and archaea) comprise the microbiota, collectively weighing around 4.5 pounds and containing over 150 times more genes than our own human genome! These microbes include a mixture of commensal (neutrally existing), symbiotic (mutually beneficial), and pathogenic (harmful to us) organisms, and can consist of any of 35,000 species known to inhabit the human gut. Every person’s gut contains approximately 400 to 1,500 different species of the possible 35,000 different microorganisms that are well adapted to survive in the gastrointestinal tract, although about 99 percent of those microorganisms come from thirty to forty species of bacteria. Our guts are inhabited by other microorganisms besides bacteria, including archaea (similar to bacteria), viruses, and single-cell eukaryotes (like yeast).
The term gut microbiome is often used a catch-all term to describe the gut microbiota plus its metabolome (the collection of biologically active molecules within and produced by our gut microbes), but microbiome technically refers to the amazing collection of genes that our gut microbiota have. The contribute 3.3 million genes whereas humans only have about 23,000 genes. This is important because our gut microbiota regulate many aspect of human health via their genetic contribution. While gut microbiome, microbiota and metabolome are often used interchangeably, it’s important to note that these three terms all describe different aspects of the microbial community in our guts. For the sake of clarity, I will use the term microbiota when referring to the collection of microbes in our guts and the term microbiome when referring to the ecosystem as a whole.
Amazingly, the gut microbiome wasn’t even widely recognized to exist until the late 1990s!
The Diverse Roles of Our Gut Microbiota
Our gut microbiota help us digest food, produce chemicals that improve the health of the cells that form the gut barrier, and directly regulate the immune system, and they can even influence brain health by producing neuroactive chemicals that are absorbed into the bloodstream and travel to the brain. A healthy diversity of the right kinds of microorganisms in the gut is one of the most fundamental aspects of good health.
The gut microbiome performs diverse functions essential to our health. Perhaps best understood is their role in digestion. Our gut microbiota have enzymes that break down certain types of sugars, starches, and fiber from foods so that we can digest them and absorb their nutrients. Bacteria also ferment fiber in our digestive tracts, producing short-chain fatty acids—such as acetic acid, propionic acid, and butyric acid—which are extremely beneficial energy sources for the body and are essential for regulating metabolism. These short-chain fatty acids also aid in the absorption of minerals such as calcium, magnesium, copper, zinc, and iron. Our gut bacteria aid in the absorption of minerals in other ways too. They degrade minerals complexing with phytate (an “antinutrient” present to varying degrees in all plant-based foods that binds minerals and makes them less absorbable; see for example Is Oxalate Sensitivity Real? and Nuts and the Paleo Diet: Moderation is Key), making those minerals available for absorption. Our gut bacteria also synthesize vitamins, B and K vitamins in particular, which our bodies then absorb (and which provide us with important micronutrients that we may not get enough of otherwise). Gut bacteria may also play a key role in facilitating absorption of dietary fatty acids, thereby also increasing absorption of important fat-soluble vitamins like A, E, D, and K (although the results of this cutting-edge research have yet to be confirmed in humans). Gut bacteria can also ferment proteins, producing branched-chain amino acids, well known to be important for muscle recovery and athletic performance.
Our gut bacteria also directly control the integrity of the gut barrier by regulating important tight junction proteins (claudin-2, occludin, cingulin, ZO-1, ZO-2) between the gut epithelial cells (see What Is A Leaky Gut? (And How Can It Cause So Many Health Issues?)). These effects aren’t limited to the gut either: recent studies have shown that our gut bacteria can regulate the permeability of epithelial barriers elsewhere in the body , including the blood-brain barrier. Yes, our gut bacteria control how leaky the blood-brain barrier is, again through regulating important tight junction proteins ( in this case, claudins, tricellulin, and occludin). There’s also an indirect effect on gut barrier integrity via modulation of serotonin (which regulates gastric motility) and Toll-like receptors (TLRs) which are important for antigen presentation by dendritic cells and macrophages to the adaptive immune system.
The microorganisms in our guts help to maintain the delicate balance required by our immune systems, keeping the various populations of immune cells in check and modulating their activity. Achieving a healthy balance in the immune system is therefore reliant on having a healthy population of gut microbiota, growing in the correct numbers in the correct locations and with appropriate diversity. In fact, let’s dig into the details on this role…
Our Gut Microbiome and the Immune System
Let’s first review the key players of the adaptive immune system, so we can understand just how vital a healthy, diverse gut microbiota is for immune function. (For more details, see The Paleo Approach)
The adaptive immune system is the part of the immune system that attacks on invading organisms (pathogens) with specificity, meaning its attacks are targeted for exactly that specific virus, bacteria, fugus or parasite that is infecting us. It also remembers invaders (this is called immunological memory) so that it responds more intensely and quickly for subsequent infections. The adaptive immune system is why vaccines protect us against infection and why we get chicken pox only once. The adaptive immune system also tailors responses to eliminate specific pathogens or pathogen-infected cells in the most effective and efficient way possible. (Contrast this to the innate immune system which is like our immune system’s first-responders; they’re fast to mobilize but can’t attack with much specificity. The innate immune system is responsible for detecting a foreign invader in the first place and then recruiting the adaptive immune system to help fight them off.)
There are two main cell types that drive adaptive immune responses: B cells (which produce antibodies) and T cells (many of which act like the middle management of the immune system).
There are a variety of different subtypes of T cells, each with it’s own function in the adaptive immune system. Among these are the helper T cells, whose job it is to control the actions of most other cell types in the immune system (hence the middle management metaphor). Some drive the immune system and inflammation, and some suppress and regulate the immune system, effectively turning off inflammation when the pathogen is vanquished. The important helper T cells for driving the immune system and inflammation are Th1, Th2, Th9, Th17, and Th22 cells). Th1 cells recruit and regulate nonspecific immune cells, such as macrophages, and secrete cytokines that stimulate T cells to mature into cytotoxic T cells. Th2 cells activate B cells (which then divide rapidly and secrete antibodies). Th9 cells are similar to Th2 cells (they are activated by different cytokines) and are important for host defense against parasitic infections (specifically helminth worms), but are also implicated in the development of chronic allergic inflammation, airway remodeling such as in asthma, and autoimmune disease. Th17 cells are similar to Th1 cells (they secrete different cytokines), are highly inflammatory, and are activated in response to certain bacteria and parasites. Excessive numbers of activated Th17 cells are present and probably responsible for tissue damage in some autoimmune diseases, including rheumatoid arthritis, multiple sclerosis, and inflammatory bowel disorders. There is also some evidence that Th17 cells may have a regulatory function similar to Th3 cells or Tr1 cells (see below), but the research on this isn’t conclusive. Th22 cells are also similar to Th1 cells (they secrete different cytokines than Th1 and Th17 cells) and have been implicated in inflammatory skin disorders such as psoriasis, atopic eczema, and allergic contact dermatitis.
There are also helper T cells that are immune modulators: their job it is to help suppress the immune system. Th3 cells (also known as adaptive regulatory T cells or induced regulatory T cells) protect the lining of the gut (the gut mucosa, or mucosal barrier of the gut) from nonpathogenic antigens (foreign substances other than viruses, bacteria, fungi, and parasites). Th3 cells also suppress Th1 and Th2 cells, making Th3 cells important immune modulators. Tr1 cells (also called type 1 regulatory T cells), which are similar to Th3 cells (they secrete different cytokines than Th3 cells), control the activation of memory T cells and suppress Th1- and Th2-mediated immune responses to pathogens, tumors, and to “self.”
Regulatory T cells are another type of T cell (not a helper T cell) that are crucial for regulating the adaptive immune system. These cells suppress the activity of immune and inflammatory cells to shut down T-cell-mediated immunity toward the end of an immune reaction. Their immune modulating activity extends to the innate immune system as regulatory T cells can also suppress activation of dendritic cells. Regulatory T cells maintain “immune tolerance,” or the process by which the immune system tolerates and chooses not to attack an antigen (which is important during pregnancy, for example). Beyond this, regulatory T cells have the critical job of suppressing the activity of any T cells that recognize self and therefore might attack healthy cells in the body. A lack (or perhaps reduced ability) of regulatory T cells is thought to be crucial the development of autoimmune disease. Cytokines produced by Th3 cells may be important in the activation of regulatory T cells.
Summary: there are many types of immune cells that work together like instruments in an orchestra to fight off an invading pathogen. When the immune system isn’t balanced (as in cancer, autoimmune disease, allergic diseases, and all situations in which systemic (bodywide) inflammation is present which really just means all chronic diseases), it’s as though the instruments aren’t in tune and are all playing different pieces. That cacophony results in an immune system that both fails at its primary role and also damages us in a variety of different ways. The gut microbiome acts as a conductor, helping to tune each instrument and making sure that the entire orchestra is playing the right piece.
A healthy gut microbiome is critical for the development and maturation of the immune system, modulating nearly every aspect of the adaptive immune system and even some of the innate immune system. For example, a complete lack of gut microbiota is known to result in severe deficiencies of most helper T cell subsets, but an increase of Th2 cells. Some bacterial components are known to balance Th1, Th2, and Th3 cell populations through regulation of dendritic cell activation (increasing or decreasing dendritic cell activation depending on the circumstance). Some bacterial components stimulate the production of Th17 cells, some modulate the activation of natural killer cells (innate immune system cells), some influence the interaction between antigen receptors on the immune cell surfaces and the antigens themselves. Probiotic bacteria not only keep the immune system in check during times of health, but also help control the immune defense against invading pathogens, for example, by stimulating the production of antibodies against the foreign microorganism.
Different bacterial components modulate different aspects of the immune system, including modulating/regulating all of the following:
- gene expression of cytokines (chemical messengers of inflammation, including IL-10, IL-22, IL-1β, IFN-γ, TGF-β1)
- production and activity of regulatory T cells
- number and activity of IgA-secreting plasma cells in the gut lining
- the balance between Th1, Th2, and Th3 cell populations via regulation of dendritic cell activation
- production of Th17 cells
- activation of natural killer cells
- the interaction between antigen receptors on the immune cell surfaces and the antigens themselves (via Toll-like receptors, TLRs)
- the production of antibodies against foreign microorganisms
For those of you who nerd out on the details of immune function like I do, you’re reading that list and thinking “woah, our gut microbiome is basically the managers of our entire immune system!”. For those of you who read that list and start going cross-eyed, the take-home message is that our gut microbiome controls virtually every aspect of how our immune system functions. Given that inflammation is part of the pathogenesis of all chronic illness, it’s no wonder we now have conclusive links between all chronic illness and irregularities in our gut bacteria.
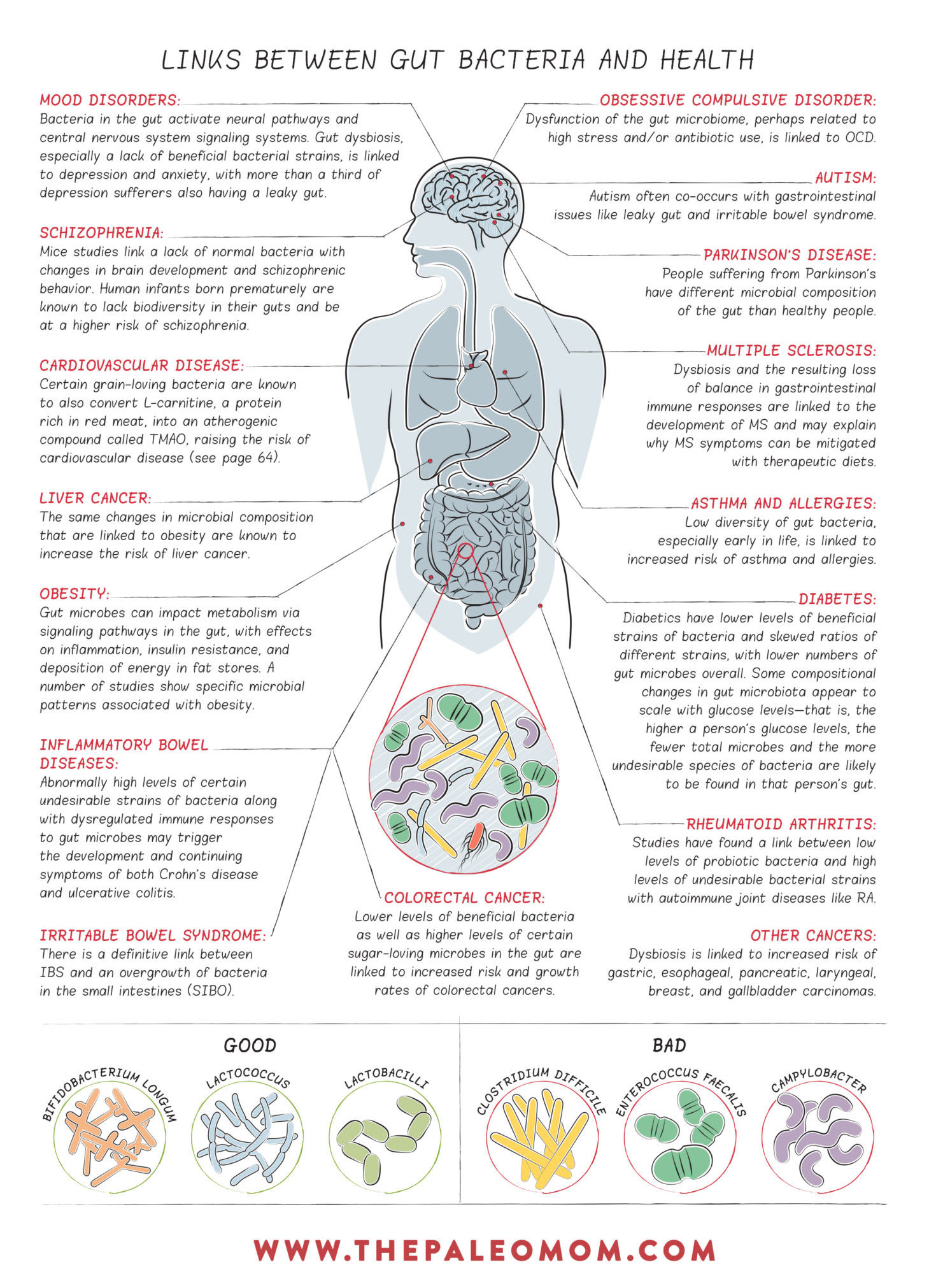
Gut Dysbiosis and Chronic Disease
Gut dysbiosis is a general term that refers to any abnormality in our gut microbiota. This includes too many or too few microorganisms growing in the various sections of the gastrointestinal tract, the wrong kinds of microorganisms or the wrong balance between the different populations of microorganisms, and microorganisms in the wrong place. Any of these situations can have profound impacts on our digestion, gut barrier health, and the modulation of our immune systems.
One common form of gut dysbiosis is overgrowth of bacteria or yeast in the small intestine. This is referred to as small intestinal bacterial overgrowth, or SIBO, (this term does apply to yeast overgrowth) and it is now believed to be the cause of irritable bowel syndrome (or at least some forms of IBS, which is probably a collection of disorders that have yet to be sorted out).
Importantly, gut dysbiosis is strongly linked to chronic disease. In fact, a link has been found in every chronic disease in which a connection to gut bacteria has been investigated.
What Does a Healthy Gut Microbiome Look Like?
Diversity is considered the number one hallmark of a healthy gut microbiome.
When the microbiota of people living in Western cultures were analyzed in comparison with those of people living in rural settings who had hunter-gatherer lifestyles and with those of wild primates like chimpanzees, Western-culture gut microbiota were found to be significantly lacking in both richness and biodiversity. This is directly attributable to diets high in industrially processed foods (which are also low in fiber), which don’t supply enough nutrition for our microbiota to thrive. Interestingly, there is even less diversity of gut bacteria in obese people than in lean people: more food does not equal more nutrition, and the worse our diet, the more our gut microbiota suffer.
In the adult human gut, two phyla (the taxonomic category right below “kingdom”) dominate: Bacteroidetes and Firmicutes. These are present in every human gut, and much smaller proportions of the phyla Actinobacteria, Proteobacteria, Verrucomicrobia, and Fusobacteria can also be present. While there are literally thousands of species of bacteria belonging to each of these phyla (including ones that are probiotic, commensal and pathogenic), it’s useful to look at some of the broad strokes when it comes to this birds-eye look at the gut microbiom.
Bacteroidetes Phylum: Bacteroidetes is one of the two most abundant phyla in the human gut microbiome (the other being Firmicutes). This phyla is relatively less susceptible to perturbations than Firmicutes and Proteobacteria, and all of its members are Gram-negative and nonsporeforming. Bacteroidetes appear strongly implicated in weight maintenance and obesity, with a higher predominance (relative to Firmicutes) being associated with significant weight loss, and a lower predominance found in obese individuals. (The obesity link is potentially due to more efficient energy extraction from carbohydrates when the Firmicutes/Bacteroidetes ratio is high, leading to an increased energy balance.) Due to its dominance in the gut microbiome, as well as its extensive positive interactions with other taxa, Bacteroidetes fits the criteria for “foundational taxon.”
Firmicutes Phylum: Along with Bacteroidetes, Firmicutes are one of the two most abundant phyla in humans, and compared to Bacteroidetes is relatively susceptible to perturbations. This phyla is represented mostly by lactic acid bacteria (such as Lactobacillus and Enterococcus, as well as Clostridium). Relatively lower levels are found in diabetics compared to nondiabetics, and lower levels are also found in patients with Crohn’s disease or IBD. A higher proportion of Firmicutes is associated with obesity, possibly due to the bacteria in this phylum increasing the efficiency of energy extraction from carbohydrates. The story here is complex though, because the gut microbiota of hunter-gatherers are dominated by Firmicutes and these bacteria dominate when diets are rich in vegetables.
Actinobacteria Phylum: Although this phylum comprises a very small proportion of the gut microbiome, it fits the criteria for “keystone taxon” due to its positive association with microbial diversity and high level of ecological connectedness. All Actinobacteria members are gram-positive, nonmotile, nonsporulating, and non-gas-producing anaerobes, and the phyla as a whole is relatively stable and resistant to perturbations.
Proteobacteria Phylum: The Proteobacteria phylum is gram negative and relatively less stable than Bacteroidetes and Actinobacteria. Most of the known pathogenic bacteria in humans belong to this phylum, and some evidence suggests that Proteobacteria members may play a key role in IBD. Proteobacteria members reside within the mucus layer in the colon and can use mucus as an energy source.
Verrucomicrobia Phylum: This phylum contains only a handful of described species, but some of those species are extremely important—namely Akkermansia muciniphilia, a major player in immune signaling and chronic disease.
How to Support a Healthy Gut Microbiome
Diet is the single biggest influence on microbiota composition. In fact, diet is directly responsible for more than 60% of the variation in bacterial species in the gut.
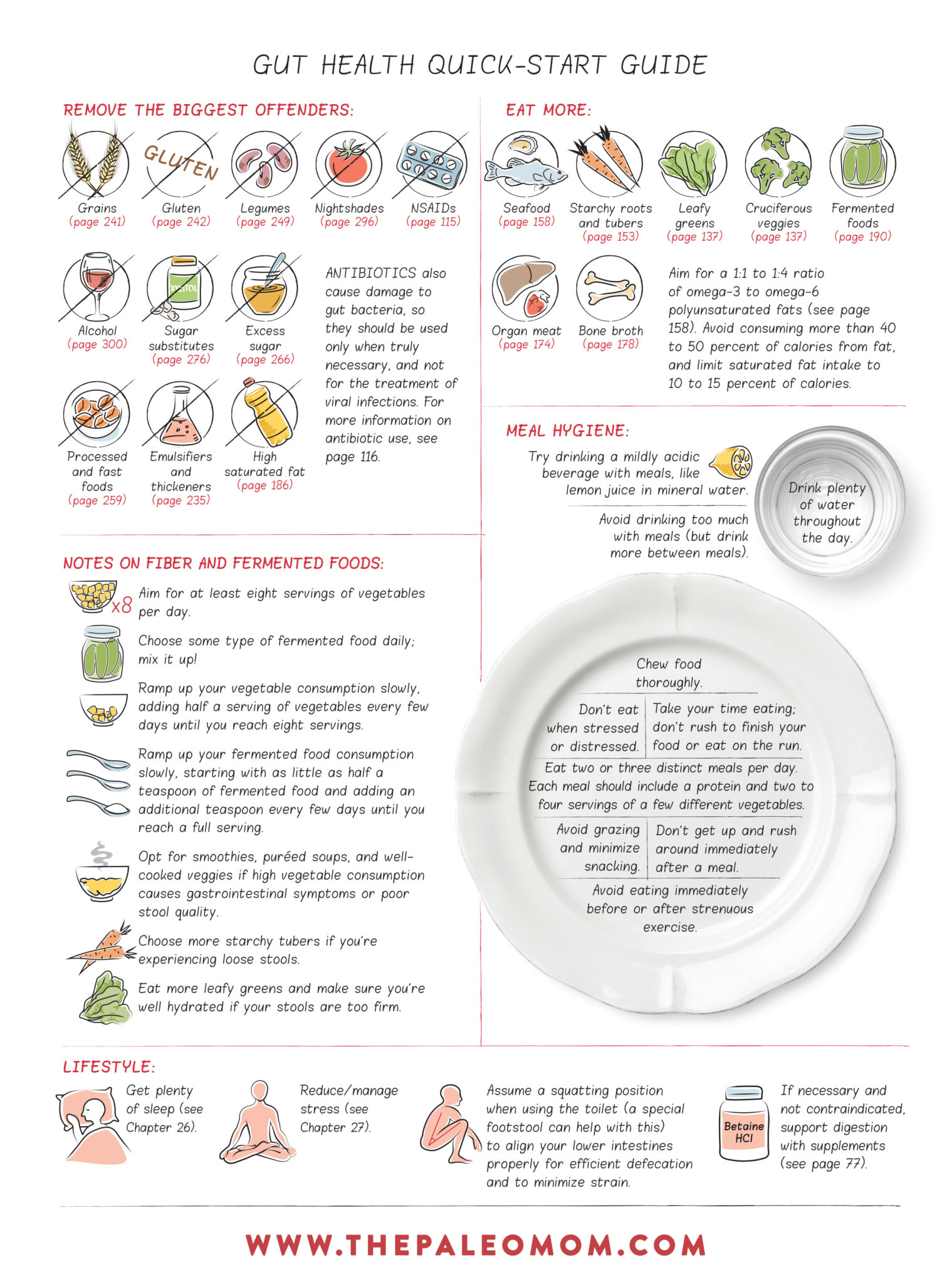
We know that inadequate fiber intake, high intake of omega-6 polyunsaturated fats (relative to omega-3s), high intake of saturated fat and low levels of vitamin D all cause a shift in the gut microbiota from probiotic to commensal, opportunitistic and pathogenic strains. In particular, inadequate fiber tends to shift the population of gut bacteria from majority Gram-positive strains (mainly those in the Firmucutes phylum) to more Gram-negative strains (mainly those in the Bacteroidetes phylum). High omega-6 fat intake depletes growth of both Firmicutes and Bacteroidetes phyla. And, high saturated fat intake skews microbiota unfavorably towards more Bilophila, Turicibacter, and Bacteroides. Vitamin D deficiency leads to shift toward pathogenic bacteria (Helicobacter, Veillonella and Erysipelotrichaceae), whereas supplementation restores levels of probiotic bacteria (Lactococcus, Akkermansia).
Some individual food compounds can also promote the growth of the wrong kinds of bacteria. Grains, dairy, legumes, nightshades, and alcohol are all known to contain compounds that can hinder the growth of beneficial strains of bacteria while supporting the growth of undesirable strains, like E. coli. These include agglutinins, prolamins, digestive enzyme inhibitors and alochols (including sugar alcohols). See Are all lectins bad? (and what are lectins, anyway?), Why Grains Are Bad-Part 1, Lectins and the Gut, Wheat and Innate Immunity, Is It Paleo? Splenda, Erythritol, Stevia and other low-calorie sweeteners and The WHYs behind the Autoimmune Protocol: Alcohol. Some emulsifiers also preferentially feed undesirable strains of bacteria (see Is It Paleo? Guar Gum, Xanthan Gum and Lecithin, Oh My!).
It’s not just a question of which kinds of bacteria our diet nourishes but also a question of bacterial metabolism (yep, the metabolome). Just as a high-sugar diet causes oxidative stress in our bodies (see Why Is Sugar Bad?), a high-sugar diet causes oxidative stress in our gut bacteria. Those bacteria adapt by altering their metabolism, which greatly affects our health.
The good news here is that the population of microbes in the gut (types, total and relative quantities, and location) adapts quite rapidly to changes in diet, in a matter of a few days to a few weeks.
- Dramatically increasing intake of fresh vegetables and fruit restores levels and diversity of probiotic species in as little as 3 to 4 days.
- Fish oil supplementation can restore levels of probiotic bacteria in about two weeks.
In fact, these are the two most important dietary factors for supporting healthy and diverse gut microbiota: eat plenty of whole vegetables and fruits, eat plenty of seafood, don’t go crazy on saturated fat. (Also see Saturated Fat: Healthful, Harmful, or Somewhere In Between?)
Lifestyle also plays a role here. Inadequate sleep, high chronic stress, living a sedentary lifestyle, and overtraining all negatively impact the microbial diversity and proportion of probiotic species in the gut. Living an active lifestyle, getting adequate sleep, and managing stress all support a healthy and diverse gut microbial community.
Exposure to probiotic organisms to inoculate the gut is also important. This is discussed in The Benefits of Probiotics and The Health Benefits of Fermented Foods.
Gut Health Quick-Start Guide
Having a healthy gut means more than just fixing a leaky one (see What Is A Leaky Gut? (And How Can It Cause So Many Health Issues?)). It also means restoring gut microbiota to the appropriate diversity, numbers, and locations—different types of bacteria grow in different amounts in different parts of the gut. In general, this means consuming a moderate amount saturated fat, a balanced ratio of omega-3 to omega-6 polyunsaturated fats, and a diversity of fiber types from a wide range of fruits and vegetables. Choosing foods as well as engaging in lifestyle choices that support gut health is a major guiding principle behind the Paleo template.
This is a guide out of my book, Paleo Principles. It represents not just the best choices for gut microbiome health but also gut barrier health, detoxification, hormone regulation and digestion (all aspects of gut health). You can also learn more in my Leaky Gut Mini-Course.
Citations
Bjarnason, I., Intestinal permeability, Gut. 1994;35(1 Suppl):S18-S22
Blaser MJ. “The microbiome revolution.” J Clin Invest. 2014 Oct;124(10):4162-5.
Purohit, V., et al., Alcohol, Intestinal Bacterial Growth, Intestinal Permeability to Endotoxin, and Medical Consequences, Alcohol. 2008;42(5):349-361
Swank GM, Deitch EA. Role of the gut in multiple organ failure: bacterial translocation and permeability changes. World J Surg 1996;20:411-417.
Cresci GA, Bawden E. Gut Microbiome: What We Do and Don’t Know. Nutr Clin Pract. 2015 Dec;30(6):734-46. doi: 10.1177/0884533615609899
Wu GD. The Gut Microbiome, Its Metabolome, and Their Relationship to Health and Disease. Nestle Nutr Inst Workshop Ser. 2016;84:103-10. doi: 10.1159/000436993.
Wu GD. Diet, the gut microbiome and the metabolome in IBD. Nestle Nutr Inst Workshop Ser. 2014;79:73-82. doi: 10.1159/000360686
John GK et al. Dietary Alteration of the Gut Microbiome and Its Impact on Weight and Fat Mass: A Systematic Review and Meta-Analysis. Genes (Basel). 2018 Mar 16;9(3). pii: E167. doi: 10.3390/genes9030167.
Tengeler AC et al. Relationship between diet, the gut microbiota, and brain function. Nutr Rev. 2018 Apr 28. doi: 10.1093/nutrit/nuy016.